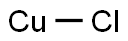Kupfer(I)-chlorid
|
|
Kupfer(I)-chlorid Eigenschaften
- Schmelzpunkt:
- 430 °C (lit.)
- Siedepunkt:
- 1490 °C (lit.)
- Dichte
- 1.15 g/mL at 20 °C
- Dampfdruck
- 1.3 mm Hg ( 546 °C)
- Brechungsindex
- 1.93
- Flammpunkt:
- 1490°C
- storage temp.
- Store at +5°C to +30°C.
- L?slichkeit
- 0.06 g/L (25°C)
- Aggregatzustand
- beads
- Farbe
- Slightly greenish-gray
- Wichte
- 4.14
- PH
- 5 (50g/l, H2O, 20℃)(slurry)
- Wasserl?slichkeit
- 0.06 g/L (25 ºC)
- Sensitive
- Air & Moisture Sensitive
- Crystal Structure
- Hexagonal, Wurtzite (Zincite) Structure - Space Group P 63mc
- crystal system
- Cube
- Merck
- 14,2660
- Solubility Product Constant (Ksp)
- pKsp: 6.76
- Space group
- F43m
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.542 0.542 0.542 90 90 90 0.1592
- Expositionsgrenzwerte
- ACGIH: TWA 1 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3
- Stabilit?t:
- Stable. Incompatible with oxidizing agents, potassium, water. Air, light and moisture sensitive.
- CAS Datenbank
- 7758-89-6(CAS DataBase Reference)
- NIST chemische Informationen
- Cuprous monochloride(7758-89-6)
- EPA chemische Informationen
- Cuprous chloride (7758-89-6)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xn,N | ||
|---|---|---|---|
| R-S?tze: | 22-50/53-51/53-36/37/38-41-37/38-38 | ||
| S-S?tze: | 26-61-60-22-39 | ||
| RIDADR | UN 3082 9/PG 3 | ||
| WGK Germany | 2 | ||
| RTECS-Nr. | GL6990000 | ||
| F | 1-8-10 | ||
| TSCA | Yes | ||
| HS Code | 2827 39 85 | ||
| HazardClass | 8 | ||
| PackingGroup | III | ||
| Toxizit?t | LD50 orally in Rabbit: 336 mg/kg |
| Bildanzeige (GHS) |
  
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
Kupfer(I)-chlorid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.R50/53:Sehr giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R51/53:Giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
S60:Dieses Produkt und sein Beh?lter sind als gef?hrlicher Abfall zu entsorgen.
S22:Staub nicht einatmen.
Chemische Eigenschaften
white or pale grey powderPhysikalische Eigenschaften
White cubic crystal which turns blue when heated at 178°C; density 4.14 g/cm3; the mineral nantokite (CuCl) has density 4.14 g/cm3, hardness 2.5 (Mohs), refractive index 1.930; melts at 430°C becoming a deep, green liquid; vaporizes around 1,400°C; vapor pressure 5 torr at 645°C and 400 torr at 1250°C; low solubility in water (decomposes partially); Ksp 1.72x10-7; insoluble in ethanol and acetone; soluble in concentrated HCl and ammonium hydroxide.The space lattice of CuCl belongs to the cubic system, and its zinc-blende structure has a lattice constant of a=0.541 nm and Cu–Cl=0.235 nm below 407°C, and it belongs to the hexagonal system and has a wurtzite structure at 407°C–422°C.The reflection peaks at room temperature are positioned at λ: 58 and 65.4 mm. The transmission peak is located at λ: 18.5 mm. The absorption spectrum near the absorption edge λ: 370.0 nm at liquid He temperature shows many structures because of exciton absorption.
Verwenden
Copper(I) chloride (CuCl) or cuprous chloride is a white powder used as an absorbing agent for carbon dioxide gas in enclosed breathing areas such as space vehicles.synthetische
Copper(I) chloride is prepared by reduction of copper(II) chloride in solution: 2CuCl2 + H2 2CuCl + 2HCl Alternatively, it can be prepared by boiling an acidic solution of copper(II) chloride with copper metal, which on dilution yields white CuCl: Cu + CuCl2 2CuCl Copper(I) chloride dissolved in concentrated HCl absorbs carbon monoxide under pressure forming an adduct, CuCl(CO). The complex decomposes on heating releasing CO. Copper(I) chloride is slightly soluble in water. However, in the presence of Cl- ion, it forms soluble complexes of discrete halogeno anions such as, CuCl2-, CuCl3 2-, and CuCl4 3-. Formation of complexes and organocopper derivatives as outlined below are not confined only to copper(I) chloride, but typify Cu+ in general. Reaction with ethylenediamine (en) in aqueous potassium chloride solution forms Cu(II)-ethylenediamine complex, while Cu+ ion is reduced to its metallic state: 2CuCl + 2en → [Cuen2]2+ + 2Cl- + Cu° It dissolves in acetonitrile, CH3CN forming tetrahedral complex ion [Cu(CH3CN)4]+ which can be precipitated with large anions such as ClO4 - or PF6- . Reactions with alkoxides of alkali metals produce yellow copper(I) alkoxides. For example, reaction with sodium ethoxide yield copper(I) ethoxide, a yellow compound that can be sublimed from the product mixture: CuCl + NaOC2H5 → CuOC2H5 + NaCl Copper(I) chloride forms complexes with ethylene and other alkenes in solutions that may have compositions such as [Cu(C2H4)(H2O)2]+ or [Cu(C2H4)(bipy)]+. (bipy = bipyridyl) Reactions with lithium or Grignard reagent yield alkyl or aryl copper(I) derivatives, respectively. Such organocopper compounds containing Cu-Cu bonds are formed only by Cu+ and not Cu2+ ions.Definition
ChEBI: An inorganic chloride of copper in which the metal is in the +1 oxidation state.Allgemeine Beschreibung
The structure of copper(I) chloride is similar to zinc-blende crystal at room temperature; the structure is wurtzite at 407 °C and at higher temperatures it forms copper(I) chloride vapor as per mass spectroscopy.Hazard
Copper(I) chloride is moderately toxic by ingestion and possibly other routes of entry into the body. The oral LD50 in mouse is reported to be 347 mg/kg; and subcutaneous LD50 in guinea pigs is 100 mg/kg.Versand/Shipping
UN2802 Copper chloride, Hazard class: 8; Labels: 8-Corrosive material.l?uterung methode
Wash the solid with ethanol and diethyl ether, then dry it and store it in a vacuum desiccator [.sterl.f Acta Chem Scand 4 375 1950]. Alternatively, to an aqueous solution of CuCl2.2H2O is added, with stirring, an aqueous solution of anhydrous sodium sulfite. The colourless product is dried at 80o for 30minutes and stored under N2. Cu2Cl2 can be purified by zone-refining [Hall et al. J Chem Soc, Faraday Trans 1 79 343 1983]. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1005 1965.]Inkompatibilit?ten
Contact with strong acids forms monovalent copper salts and toxic hydrogen chloride gas. Forms shock-sensitive and explosive compounds with potassium, sodium, sodium hypobromite, nitromethane, acetylene. Keep away from moisture and alkali metals. Attacks metals in the presence of moisture. Reacts with moist air to form cupric chloride dihydrate. May attack some metals, paints, and coatings. May be able to ignite combustible materials.Kupfer(I)-chlorid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Kupfer
Ethanol
Natriumsulfat
Natriumchlorid
Natriumsulfit
Chlor
Schwefeltrioxid
Schwefligesure
Copper(II) sulfate pentahydrate
Copper wire
Hydrogenchlorid
Kupferdihydroxid
Kupfersulfat
Schwefelsure
Downstream Produkte
5-[4-FLUORO-3-(TRIFLUOROMETHYL)PHENYL]-2-FURALDEHYDE
Natrium-1-amino-9,10-dihydro-9,10-dioxo-4-(2,4,6-trimethylanilino)anthracen-2-sulfonat
5-[2-CHLORO-4-(TRIFLUOROMETHYL)PHENYL]-2-FURALDEHYDE
Clonazepam
2-Bromchlorbenzol
4-Chlor-3-nitrotoluol
2-Chlor-1,3-dinitrobenzol
3,5-DICHLORO (1H)INDAZOLE
Di-t-butylmethylphosphine
7-CHLOROQUINOLINE
Chlortoluol (m)
Dichlor(methyl)silan
4-Chlor-N-methylanilin
Bis(trimethylsilyl)acetylen
2-(4'-Chlorbenzyl)pyridin
5-[2,6-DICHLORO-4-(TRIFLUOROMETHYL)PHENYL]-2-FURALDEHYDE
Acryloylchlorid
3-(P-CHLOROPHENYL)-3-(2-PYRIDYL)PROPYLALDEHYDE DIETHYL ACETAL
2-(3-(4-Amino-9,10-dihydro-3-sulfo-9,10-dioxoanthracen-4-yl)aminobenzolsulfonyl)vinyl)dinatriumsulfat
Phenyl-m-tolylether
2,4,5-Trifluorophenylacetic acid
3-CHLOROTHIOPHENE-2-CARBOXAMIDE
Dinatrium-4,4'-[methylenbis(4,1-phenylenimino)]bis[1-amino-9,10-dihydro-9,10-dioxoanthracen-2-sulfonat]
5-[4-(TRIFLUOROMETHOXY)PHENYL]-2-FURALDEHYDE
3-CHLORO-2-METHYLBENZONITRILE
N,N,N',N'-Tetramethyl-p-phenylendiamindihydrochlorid
1,4-Dichlornaphthalin
2-Chloro-3-methyl-5-bromopyridine
2,3-Dichlorobenzaldehyde
Dinatrium-[29H,31H-phthalocyanindisulfonato(4-)-N29,N30,N31,N32]cuprat(2-)
5-Chloro-2-fluorobenzoic acid
4-Chlor-2-(4-methoxyphenyl)anthranilsure
1,3-Dichlorbut-2-en
Hexans?ure-2-propenylester
2,4,6-Trimethoxybenzaldehyd
5-(4-Bromophenyl)furfural
2-Chloro-5-iodopyridine
16,23-Dihydronaphth[2',3':6,7]indolo[2,3-c]dinaphtho[2,3-a:2'3'-i]carbazol-5,10,15,17,22,24-hexon
Chlor(methyl)silan
Dibenzo[def,mno]chrysen-6,12-dion
Kupfer(I)-chlorid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 561)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5857 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18151 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3002 | 58 |
| Watson Biotechnology Co.,Ltd | +86-18186686046 +86-18186686046 |
sales01@watsonbiotech.cn | China | 5841 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2986 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29880 | 58 |
7758-89-6(Kupfer(I)-chlorid)Verwandte Suche:
Polyvinyl chloride
Kupfer
Acetylchlorid
Benzylchlorid
Natriumchlorid
Cholinchlorid
Kaliumchlorid
Ammoniumchlorid
Calciumchlorid
Kupferchlorid
4-(DICYANOMETHYLENE)-2-METHYL-6-(JULOLIDIN-4-YL-VINYL)-4H-PYRAN
Dikupferchloridtrihydroxid
Kupferchlorophthalocyaonin
Kupfer(II)-chlorid
Diammoniumtetrachlorocuprat
Kupfer(I)-chlorid
CHLORIDE STANDARD
- Copper(I) chloride ReagentPlus(R), purified, >=99%
- Copper(I) chloride, 99.999% trace metals basis
- Copper(I) chloride >=99.995% trace Metals basis
- Copper(I) chloride anhydrous, beads, >=99.99% trace Metals basis
- Copper(I) chloride, extra pure, 97+%
- Copper(I) chloride, extra pure, 99.99%
- Copper(I) chloride, for analysis ACS, 90+%
- Copper(I) chloride, purified, extra pure, 99%
- CUPROUS CHLORIDE 99.999%
- CUPROUS CHLORIDE REAGENT (ACS)
- Copper(I)chloride(99.999%-Cu)PURATREM
- Copper(I)chloride,anhydrous,97+%
- copper(i) chloride, acs
- CUPROUSCHLORIDE,POWDER,REAGENT,ACS
- CUPROUSCHLORIDE,TECHNICAL
- Kupfer(I)-chlorid
- Copper(I) chloride, ACS, 90+%
- Copper(I) chloride, 99% (metals basis)
- copper chloride copper (I) chloride cuprous chloride
- Cuprous Chloride, Anhydrous
- Cuprous Chloride, Powder
- Copper (I) Chloride, -80 Mesh
- Copper(I) chloride,99%,extra pure,purified
- COPPER(I) CHLORIDE, ANHYDROUS
- Copper(I) chloride, 97%+
- Copper(I)chloride,(L-A025)
- Copper(I)chloride,99.999%(metalsbasis)
- Copper(i) chloride, 99%, purified, extra pure
- Copper(i) chloride, 99.99%, extra pure
- COPPER(I) CHLORIDE, 97+%, EXTRA PURE
- Copper(I) chloride, extra pure
- Copper(I) chloride, for analysis ACS
- Chlorocopper(I)
- Nantokite
- Copper(I) chloride, 99% trace metals basis
- Copper(I) chlorideACS reagent, ≥ 90.0% (Titration)
- Copper(I) chloride, 90+%
- chloridmedny
- Copper chloride (CuCl)
- copperchloride(cucl)
- coppermonochloride
- Cu-lyt
- Cuproid
- COPPER(+1)CHLORIDE
- COPPER[1]CHLORIDE
- COPPER(I) CHLORIDE
- COPPER (I) CHLORIDE ACID
- CUPROUS CHLORIDE ACID
- cuprousdichloride
- dicopperdichloride
- COPPER(I) CHLORIDE REAGENT GRADE 97%
- COPPER(I) CHLORIDE, >=90%, A.C.S. REAGENT (NO BULK SALES ALLOWED)
- COPPER(I) CHLORIDE PURE
- CUPROUS CHLORIDE ACS REAGENT
- COPPER(I) CHLORIDE >=90% A.C.S. REAGE&
- COPPER(I) CHLORIDE, 99.995+%
- COPPER(I) CHLORIDE, ANHYDROUS, BEADS, 9&
- COPPER(I) CHLORIDE PURIFIED REAGENTPL&