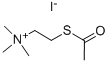2-Acetylthioethyltrimethyl-ammoniumiodid
|
|
2-Acetylthioethyltrimethyl-ammoniumiodid Eigenschaften
- Schmelzpunkt:
- 205-210 °C(lit.)
- Dichte
- 1.4885 (estimate)
- storage temp.
- 2-8°C
- L?slichkeit
- H2O: 100 mg/mL
- Aggregatzustand
- powder
- Farbe
- white
- Sensitive
- Light Sensitive & Hygroscopic
- BRN
- 3916578
- InChI
- InChI=1S/C7H16NOS.HI/c1-7(9)10-6-5-8(2,3)4;/h5-6H2,1-4H3;1H/q+1;/p-1
- InChIKey
- NTBLZMAMTZXLBP-UHFFFAOYSA-M
- SMILES
- [N+](C)(C)(C)CCSC(=O)C.[I-]
- CAS Datenbank
- 1866-15-5(CAS DataBase Reference)
- EPA chemische Informationen
- Ethanaminium, 2-(acetylthio)-N,N,N-trimethyl-, iodide (1866-15-5)
Sicherheit
| Kennzeichnung gef?hrlicher | T,Xi | ||
|---|---|---|---|
| R-S?tze: | 21-25-36/37/38 | ||
| S-S?tze: | 26-36 | ||
| RIDADR | UN 2811 6.1/PG 3 | ||
| WGK Germany | 3 | ||
| RTECS-Nr. | FZ9865000 | ||
| F | 3-8-10 | ||
| TSCA | Yes | ||
| HazardClass | 6.1 | ||
| PackingGroup | III | ||
| HS Code | 29309099 |
2-Acetylthioethyltrimethyl-ammoniumiodid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R21:Gesundheitssch?dlich bei Berührung mit der Haut.R25:Giftig beim Verschlucken.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Beschreibung
Acetylthiocholine iodide, also known as ATCI, serves as both an acetylcholinesterase (AChE) substrate and a nicotinic acetylcholine receptor (AChR) agonist. ATCI itself is a colorless and odorless compound. It exhibits solubility in both water and ethanol. It is a synthetic compound extensively employed in laboratory experiments to measure the activity of AChE, a vital enzyme responsible for the breakdown of the neurotransmitter acetylcholine (ACh) in the central and peripheral nervous systems. Additionally, it enables the evaluation of drug effects on AChE activity and facilitates investigations into the impact of toxins on the nervous system. Acetylthiocholine iodide functions as an inhibitor of AChE. By binding to the enzyme′s active site, it prevents AChE from catalyzing the breakdown of ACh. Consequently, ACh accumulates in the synaptic cleft, leading to increased neuron excitability. This inhibition of AChE by ATCI ultimately results in a higher ACh concentration within the synaptic cleft, yielding a range of physiological effects. These effects encompass heightened muscle contraction, increased heart rate and blood pressure, elevated salivation, intensified bronchial secretions, and augmented gastrointestinal activity[1].Chemische Eigenschaften
white crystalline powderVerwenden
Acetylthiocholine iodide has been used as a substrate in the preparation of acetylcholine esterase (AChE) assay working solution for AChE activity assay. It has also been used as a component in phosphate buffer (PB) for cholinesterase assay.l?uterung methode
Recrystallise the iodide from propan-1-ol (or iso-PrOH, or EtOH/Et2O) until almost colourless and dry it in a vacuum desiccator over P2O5. Its solubility in H2O is 1% w/v. A 0.075M (21.7mg/mL) solution in 0.1M phosphate buffer pH 8.0 is stable for 10-15 days if kept refrigerated. Store it away from light. It is commercially available as a 1% solution in H2O. [Ellman et al. Biochemical Pharmacology 7, 88 1961, IR: Hansen Acta Chem Scand 13 151 1959, 11 537 1957, Clin Chim Acta 2 316 1957, Ivin Zh Obshch Khim 22 267 1952, Beilstein 4 III 726, 4 IV 1585.]2-Acetylthioethyltrimethyl-ammoniumiodid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Essigs?ureanhydrid
Aceton
2-(Dimethylamino)ethanthiolhydrochlorid
2-Dimethylaminoethanol
Diethylether
Ethylacetat
Hydrogenchlorid
Natriumhydrogencarbonat
Dichlormethan
Natriumhydroxid
Thionyldichlorid
Kaliumthioacetat
Methyljodid
Thioharnstoff
Downstream Produkte
2-Acetylthioethyltrimethyl-ammoniumiodid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 287)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Suzhou BEC Biological Technology Co., Ltd. | +undefined13771885869 |
all@bek.com.cn | China | 62 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5857 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 |
bess@weibangbio.com | China | 18151 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18751 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29880 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Changzhou Ansciep Chemical Co., Ltd. | +86 519 86305871 |
sales@ansciepchem.com | CHINA | 4241 | 58 |
| HaBo Hong Kong Co., Limited. | +86-25-18512596065 |
info@habotech.com | CHINA | 933 | 58 |
| Yunbio Tech Co.,Ltd. | +86-010-60605551 +86-18046518538 |
yunbiochem@126.com | China | 321 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
1866-15-5(2-Acetylthioethyltrimethyl-ammoniumiodid)Verwandte Suche:
(Propylcarbonylthioethyl)-trimethylammoniumiodid
(2-Mercaptoethyl)trimethylammoniumiodid
Ethyltrimethylammoniumiodid
Ethylethanthioat
Cholinhydroxid
Ammoniumiodid
Choliniodid
2-Acetylthioethyltrimethyl-ammoniumiodid
2-Acetylthiopropyltrimethylammoniumiodid
Trimethyl(2-propionylthioethyl)ammoniumchlorid
- Acetylthiocholinjodid
- S-ACETYLTHIOCHOLINE IODIDE extrapure AR substrate for acetylcholinesterase
- S-Acetylthiocholine iodide,98%
- acetylthiocholinediiodide
- Ethanaminium,2-(acetylthio)-N,N,N-trimethyl-,iodide
- S-[2-(trimethyl-lambda5-azanyl)ethyl] ethanethioate hydroiodide
- s-acetylthio-choliniodide
- (2-MERCAPTOETHYL)TRIMETHYLAMMONIMUM IODIDE ACETATE
- (2-MERCAPTOETHYL)TRIMETHYLAMMONIUM IODIDE ACETATE
- 2-ACETOTHIOETHYLTRIMETHYLAMMONIUM IODIDE
- [2-(ACETYLTHIO)ETHYL](TRIMETHYL)AMMONIUM IODIDE
- 2-(THIOACETYLOXY)-N,N,N-TRIMETHYLETHANAMINIUM IODIDE
- Acetylthiocholine iodide ,98%
- Acetylthiocholine iodide,(2-Mercaptoethyl)trimethylammonium iodide acetate
- Acetylthiocholine io
- S-Acetylthiocholine iodide, 98% 1GR
- S-Acetylthiocholine iodide, 98% 5GR
- acetylthiocholine iodie
- acethylthiocholine iodide
- 2-acetylsulfanylethyl(trimethyl)azanium,iodide
- INDICATOR PAPER PH 4,5-10 100 STRIPS
- (2-mercaptoethyl)trimethyl-ammoniuiodideacetate
- 2-(acetylthio)-n,n,n-trimethyl-ethanaminiuiodide
- 2-(acetylthio)-n,n,n-trimethylethanaminiumiodide
- S-ACETYLTHIOCHOLINE IODIDE
- N-(2-MERCAPTOETHYL)TRIMETHYLAMMONIUM IODIDE ACETATE
- THIOCHOLINE IODIDE ACETATE
- Acetylthiocholine iodide≥ 98% (HPLC)
- ACETYLTHIOCHOLINE IODIDE
- 2-(acetylsulfanyl)-N,N,N-trimethylethanaminium iodide
- AcetylthiocholineIodide>
- S-Acetylthiocholine Iodide extrapure AR, 99%
- 2-acetylsulfanylethyl(trimethyl)azanium
- Iodine thioacetylcholine
- 2-(Acetylthio)-N,N,N-trimethylethan-1-aminium iodide
- Kamovsky-roots solution
- 1866-15-5
- CH3COSCH2CH2NCH33I
- C7H16SNOI
- C7H16NOSI
- C7H16INOS
- Substrates by Enzyme
- Acetylcholinesterase
- Enzyme Substrates
- Enzymes, Inhibitors, and Substrates
- Ligand-Gated Ion Channels
- Ion Channels
- Nicotinic Acetylcholine Receptor Modulators
- Cell Biology
- Cell Signaling and Neuroscience
- Ammonium Iodides (Quaternary)
- Quaternary Ammonium Compounds
- BioChemical
- Biochemicals and Reagents
- susbtrate
- Substrates
- Ammonium Iodides (Quaternary)
- Quaternary Ammonium Compounds