Thioharnstoff Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
WEISSE KRISTALLE ODER PULVER.
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen unter Bildung giftiger Rauchemit Stickstoffoxidenund Schwefeloxiden. Reagiert sehr heftig mitAcrolein, starken S?urenund starken Oxidationsmitteln.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK: Sensibilisierung der Haut; Photosensibilisierung; Krebserzeugend Kategorie 3B; (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation des Aerosols und durch Verschlucken.
INHALATIONSGEFAHREN
Verdampfung bei 20°C vernachl?ssigbar; eine gesundheitssch?dliche Partikelkonzentration in der Luft kann jedoch schnell erreicht werden.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt die Augen.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Kontakt kann zu Hautsensibilisierung führen. M?glich sind Auswirkungen auf die Schilddrüse. M?glicherweise krebserzeugend für den Menschen.
LECKAGE
Gefahrenbereich verlassen! Fachmann zu Rate ziehen! NICHT in die Kanalisation spülen. Verschüttetes Material in abgedeckten Beh?ltern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste sorgf?ltig sammeln. An sicheren Ort bringen. NICHT in die Umwelt gelangen lassen. Chemikalienschutzanzug. Pers?nliche Schutzausrüstung: Atemschutzger?t, P2-Filter für sch?dliche Partikel.
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.
R40:Verdacht auf krebserzeugende Wirkung.
R51/53:Giftig für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R63:Kann das Kind im Mutterleib m?glicherweise sch?digen.
S-S?tze Betriebsanweisung:
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
Beschreibung
Thiourea appears as white crystal/powder, is combustible, and on contact with fire, gives
off irritating or toxic fumes/gases. Thiourea is a reducing agent used primarily in the production
of bleached recycled pulp. In addition, it is also effective in the bleaching of stone
groundwood, pressurised groundwood. Thiourea undergoes decomposition on heating
and produces toxic fumes of nitrogen oxides and sulphur oxides. It reacts violently with
acrolein, strong acids, and strong oxidants. The main application of thiourea is in textile
processing and also is commonly employed as a source of sulphide. Thiourea is a precursor
to sulphide to produce metal sulphides, for example, mercury sulphide, upon reaction
with the metal salt in aqueous solution. The industrial uses of thiourea include production
of flame-retardant resins and vulcanisation accelerators. Thiourea is used as an auxiliary
agent in diazo paper, light-sensitive photocopy paper, and almost all other types
of copy paper. Thiourea is used in many industrial applications, including as a chemical
intermediate or catalyst, in metal processing and plating, and in photoprocessing.
Chemische Eigenschaften
Thiourea consists of colorless, lustrous crystals or powder with a bitter taste.
Verwenden
The product is wildly used in pharmaceutical industry, agricultural, chemicals, metallurgical industry, petroleum and so on. It is also main material for producing thiourea dioxide(CH1N2O2S).
Definition
ChEBI: The simplest member of the thiourea class, consisting of urea with the oxygen atom substituted by sulfur.
synthetische
Thiourea is manufactured by heating ammonium thiocyanate at 140-145??C
for about 4 hours; equilibrium is established when about 25% of the
thiocyanate is converted to thiourea.Thiourea may also be prepared by the interaction of cyanamide and hydrogen sulphide:
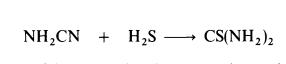
Thiourea closely resembles urea in that reaction with formaldehyde gives
methylol derivatives and then resinous condensates which on continued
heating yield network structures. Thiourea-formaldehyde resins are slower
curing than urea-formaldehyde resins and the hardened products are more
brittle and more water-resistant. At one time thiourea-formaldehyde resins
were added to urea-formaldehyde resins to give mouldings and laminates
with improved water-resistance. These mixed resins have now been largely
superseded by melamine-formaldehyde resins which give products with better
resistance to heat.
Vorbereitung Methode
Thiourea is formed by heating ammonium thiocyanate at 170 °C (338 °F). After about an hour, 25% conversion is achieved. With HCl, thiourea forms thiourea hydrochloride; with mercuric oxide, thiourea forms a salt; and with silver chloride, it forms a complex salt.
Allgemeine Beschreibung
White or off-white crystals or powder. Sinks and mixes with water.
Air & Water Reaktionen
Water soluble.
Reaktivit?t anzeigen
Thiocarbamide is a white crystalline material or powder, toxic, carcinogenic. When heated to decomposition Thiocarbamide emits very toxic fumes of oxides of sulfur and oxides of nitrogen. Violent exothermic polymerization reaction with acrylaldehyde (acrolein) [MCA SD-85, 1961], violent decomposition of the reaction product with hydrogen peroxide and nitric acid [Bjorklund G. H. et al., Trans. R. Soc. Can.,1950, 44, p. 28], spontaneous explosion upon grinding with potassium chlorate [Soothill, D., Safety Management, 1992, 8(6), p. 11].
Hazard
A questionable carcinogen. May not be
used in food products (FDA); skin irritant (allergenic).
Health Hazard
The acute oral toxicity of thiourea in mostanimals is of low order. The oral LD50 values reported in the literature show variation.Symptoms of chronic effects in rats includebone marrow depression and goiters. Administration of 32.8 mol of thiourea in chickembryos on day 17 of incubation resultedin the accumulation of parabronchial liquidin those embryos (Wittman et al. 1987). Theinvestigators have attributed such changes tothe toxic effects of thiourea, rather to than aretardation of pulmonary development.Dedon and coworkers (1986) observed thepossible protective action of thiourea againstplatinum toxicity. Thiourea and other sulfur-containing nucleophiles have the ability tochelate and remove platinum from biochemical sites of toxicity.Oral administration of thiourea resultedin tumors in the liver and thyroid in rats.It is carcinogenic to animals and has shownsufficient evidence.
Brandgefahr
Noncombustible solid. There is no report of
any explosion resulting from reactions of
thiourea. Small amounts of thiourea in contact with acrolein may polymerize acrolein,
which is a highly exothermic reaction.
Landwirtschaftliche Anwendung
Thiourea is a sulphur analogue of urea. It is a crystalline
and colorless solid which is relatively insoluble in water.
Thiourea, capable of breaking the dormancy of seeds, is
used to stimulate seed germination. Seeds are soaked for
less than 24 hours before planting.
Kontakt-Allergie
Thiourea is used as a cleaner agent for silver and cop-
per, and as an antioxidant in diazo copy paper. It can
induce (photo-) contact dermatitis.
m?gliche Exposition
Thiourea is used as rubber antiozonant, toning agent; corrosion inhibitor; and in pharmaceutical manufacture; in the manufacture of photosensitive papers; flame-retardant textile sizes; boiler water treatment. It is also used in photography; pesticide manufacture; in textile chemicals.
Carcinogenicity
Thiourea is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental
animals.
Versand/Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
l?uterung methode
Crystallise thiourea from absolute EtOH, MeOH, acetonitrile or water. Dry it under vacuum over H2SO4 at room temperature. [Beilstein 3 IV 342.]
Inkompatibilit?ten
Dust may form explosive mixture with air. Reacts violently with acrolein, strong acids (nitric acid). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Thioharnstoff Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
2-CHLORO-5-NITROTHIAZOLE
4-(4-CHLOROPHENYL)PYRIMIDINE-2-THIOL
N-(Aminothioxomethyl)acetamid
2-AMINO-6-METHYL-PYRIMIDINE-4-THIOL
2-Amino-((1-carboxy-1-methyl ethoxy)imino)-4-thiazoleacetic acid
6-AMINO-2-METHYLTHIO-3-METHYLURACIL
4,6-Dimethyl-2-methylmercapyrimidine
2-Amino-4-methyl-5-acetylthiazole
ETHYL 2-CHLORO-4-METHYL-1,3-THIAZOLE-5-CARBOXYLATE
Ethyl 4-amino-2-(ethylthio)-5-pyrimidinecarboxylate
9,10-Dimethylanthracen
4-AMINO-2-MERCAPTOPYRIMIDINE-5-CARBONITRILE
4,6-DIMETHYL-2-THIOPYRIMIDINE
Ethyl 2-amino-4-phenyl-5-thiazolecarboxylate
Oxfendazol
AMICARTHIAZOL
ETHYL 2-(ETHYLTHIO)-4-HYDROXYPYRIMIDINE-5-CARBOXYLATE
2-Imino-1,3-thiazol-4-on
Ethyl 2-amino-4-methylthiazole-5-carboxylate
2-Thiobarbitursure
(Z)-2-Amino-α-(methoxyimino)thiazol-4-essigsure
2-Amino-5-methylthiazole
2-Methylpropan-1-thiol
Ethyl-(Z)-2-amino-α-(hydroxyimino)thiazol-4-acetat
METHYL 2-CHLORO-4-THIAZOLECARBOXYLATE
4,5-Dihydrothiazol-2-aminmonohydrochlorid
1,5,6,7-Tetrahydro-6-thioxo-4H-pyrazolo[3,4-d]pyrimidin-4-on
4,6-Diaminopyrimidin-2-thiol
Methylene dithiocyanate
Octan-1-thiol
4-AMINO-2-(METHYLTHIO)-6-PYRIMIDINOL
4-Amino-6-hydroxy-2-mercaptopyrimidine monohydrate
Guanidinoessigsure
Prazosin
2-Aminothiazol-4-essigsure
5-METHOXY-2-SULFANYL-4-PYRIMIDINOL
Cyclohexanthiol
2-thiouracil
1-Dodecanethiol
5-ETHYL-2-THIOURACIL

