Natriumacetat Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
WEISSES, HYGROSKOPISCHES, KRISTALLINES PULVER
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen und bei Kontakt mit starken S?uren unter Bildung von Essigs?urerauchen. Reagiert heftig mit starken Oxidationsmitteln. Schwache Base in w?ssriger L?sung.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK nicht festgelegt (DFG 2006).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Verschlucken.
INHALATIONSGEFAHREN
Nur ungenügende Angaben vorhanden über die Geschwindigkeit, mit der eine gesundheitssch?dliche Konzentration in der Luft erreicht wird.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Der Stoff ist schwach reizend auf die Augen und die Haut.
LECKAGE
Pers?nliche Schutzausrüstung: Atemschutzger?t, P1-Filter für inerte Partikel. Verschüttetes Material in abgedeckten Beh?ltern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste mit viel Wasser wegspülen.
S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
Beschreibung
Sodium acetate (CH3COONa) is the sodium salt of acetic acid. It appears as a colorless deliquescent salt with a wide range of applications. In industry, it can be used in textile industry to neutralize sulfuric acid waste streams and as a photoresist upon using aniline dyes. In concrete industry, it can be used as a concrete sealant to mitigate the water damage. In food, it can be used as a seasoning. It can also be used as a buffer solution in lab. In addition, it is also used in heating pads, hand warmers and hot ice. For laboratory use, it can be produced by the reaction between acetate with the sodium carbonate, sodium bicarbonate and sodium hydroxide. In industry, it is prepared from the glacial acetic acid and sodium hydroxide.
Chemische Eigenschaften
Sodium acetate, CH3COONa, also abbreviated NaOAc , also sodium ethanoate, is the sodium salt of acetic acid, was made by the reaction of acetic acid with sodium carbonate. It is soluble in water but less so in alcohol. This colourless salt has a wide range of uses. Sodium acetate was used as a pH modifier for toning baths.
Physikalische Eigenschaften
Anhydrous salt is a colorless crystalline solid; density 1.528 g/cm
3; melts at 324°C; very soluble in water; moderately soluble in ethanol. The colorless crystalline trihydrate has a density 1.45 g/cm
3; decomposes at 58°C; is very soluble in water; pH of 0.1M aqueous solution is 8.9; moderately soluble in ethanol, 5.3 g/100mL.
Occurrence
Acetic acid or acetates are present in most plant and animal tissues in small, but detectable amounts
Verwenden
Used as buffers.
Acidity regulation (buffering)
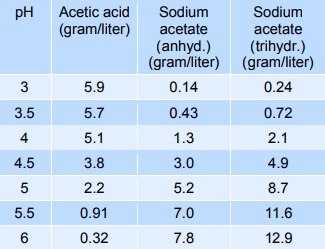
Sodium acetate mixed with acetic acid forms a pH buffer, which can be used to stabilise the pH of foods in the pH-range from 3 to 6. The table below gives indicative values of the composition needed to give a certain pH. The mixtures below can be diluted at least 10 times with minimum effect on pH, however, the stability decreases.
synthetische
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate.
NaOH + CH3COOH → CH3COONa + H2O
Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O
Definition
ChEBI: Sodium acetate is an organic sodium salt. It contains an acetate.
Synthese
For laboratory use, sodium acetate is very inexpensive, and is usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid (ethanoic acid) with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate and water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized). This is the well-known "volcano" reaction between baking soda (sodium bicarbonate) and vinegar.
CH
3COOH + NaHCO
3 → CH
3COONa + H
2O + CO
2 Industrially, sodium acetate is prepared from glacial acetic acid and sodium hydroxide.
CH
3COOH + NaOH → CH
3COONa + H
2O.
Reaktionen
Sodium acetate can be used to form an ester with an alkyl halide such as bromo ethane:
CH
3COONa + Br CH
2CH
3→ CH
3COOCH
2CH
3+ NaBr
Caesium salts catalyze this reaction.
Allgemeine Beschreibung
Sodium Acetate is reported to inhibit the growth of
Listeria monocytogenes.
Reaktivit?t anzeigen
When sodium acetate reacts with strong acids, irritating, noxious vapors of acetic acid are usually produced. Sodium acetate is sufficiently basic to catalyze the violent polymerization of diketene, perhaps as well as other reactive dimers that are susceptible to polymerization in the presence of a mild base.
Biologische Aktivit?t
Commonly used laboratory reagent
Sicherheitsprofil
Poison by intravenous route. Moderately toxic by ingestion. A skin and eye irritant. Migrates to food from packagmg materials. Violent reaction with F2, m03, diketene. When heated to decomposition it emits toxic fumes of Na2O.
l?uterung methode
Crystallise it from acetic acid and keep it under vacuum for 10hours at 120o. Alternatively, it is crystallised from aqueous EtOH, as the trihydrate. This material can be converted to anhydrous salt by heating slowly in a porcelain, nickel or iron dish, so that the salt liquefies. Steam is evolved and the mass again solidifies. Heating is now increased so that the salt melts again. (NB: if it is heated too strongly, the salt can char; avoid this.) After several minutes, the salt is allowed to solidify and is cooled to a convenient temperature (in a desiccator) before being powdered and bottled. The water content should now be less than 0.02%. [Beilstein 2 II 113, 2 III 184, 2 IV 109.]
Natriumacetat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Ibudilast
7H-Dibenzo[c,g]carbazol
4-METHYL-2-METHYLSULFANYL-PYRIMIDINE-5-CARBOXYLIC ACID ETHYL ESTER
Dehydrocholsure
CHEMBRDG-BB 4023079
ethyl 2-(methylsulfanyl)-4-(trifluoromethyl)pyrimidine-5-carboxylate
2-(Methylthio)ethanol
3-Pyridazinecarboxylic acid
Citronellylacetat
p-Menth-1-en-8-ylacetat
3-(3-Nitrophenyl)propionic acid
endo-8-Isopropyl-8-azabicyclo[3.2.1]octan-3-ol
5-NITRO-2-FURONITRILE
6-Methyl-1,2,4-triazin-3,5-diol
Menthylacetat
Natriumhydrogendi(acetat)
2-Nitro-N-phenylanilin
4-Aminotetrahydropyran hydrochloride
Cinnamylacetat
3-NITRO-PYRIDINE-2-CARBOXYLIC ACID
3-Acetyl-4-hydroxy-2-benzopyron
1,2-Epoxycyclooctan
Trypsin Inhibitor
Levosimendan
Trinatrium-4-hydroxy-4-(4-anilino-5-sulfonato-1-naphthylazo)naphthalin-2,7-disulfonat
Natrium-[2S-(2α,5α,6β)]-3,3-dimethyl-6-[[(5-methyl-3-phenylisoxazol-4-yl)carbonyl]amino]-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-2-carboxylat
p-Methoxybenzylacetat
ETHYNYLFERROCENE
L-Menthylacetat
3-Acetoxybenzofuran
5-Formylfurfurylacetat
Phospholan (ISO)
Anthra[9,1,2-cde]benzo[rst]pentaphen-5,10-dion, Amino-, Reaktionsprodukte mit 1-Amino-9,10-anthracendion und Tetrabrom-8,16-pyranthrendion
Menadioldi(acetat)
Synthetic greasing agent
METHYL2-HEXENOATE
N,N-Dimethyl-p-phenyl-azoanilin
3-(4-FLUOROPHENYL)-1H-PYRAZOLE-4-CARBALDEHYDE
Tri(dimethylamino)benzotriazol-1-yloxyphosphoniumhexafluorphosphat
Tris(dibenzylideneacetone)dipalladium

