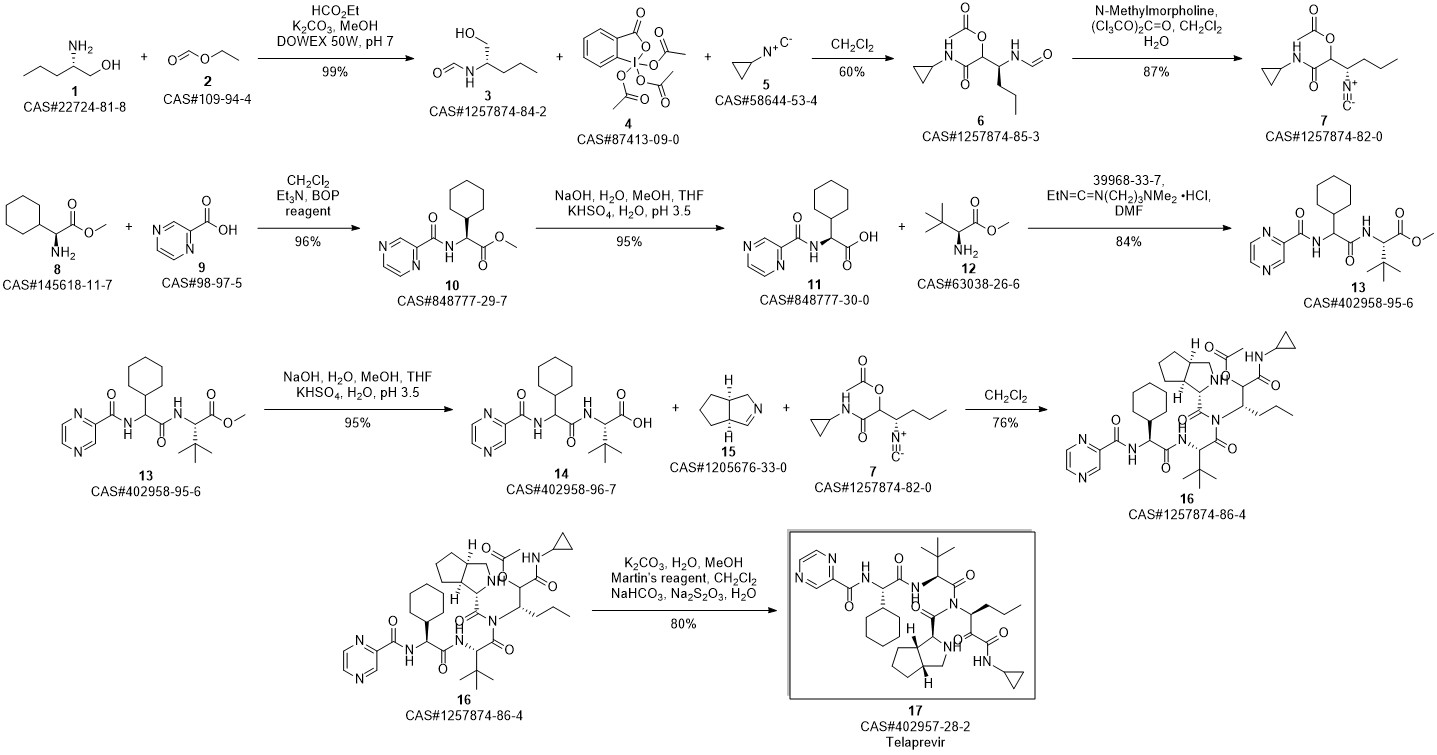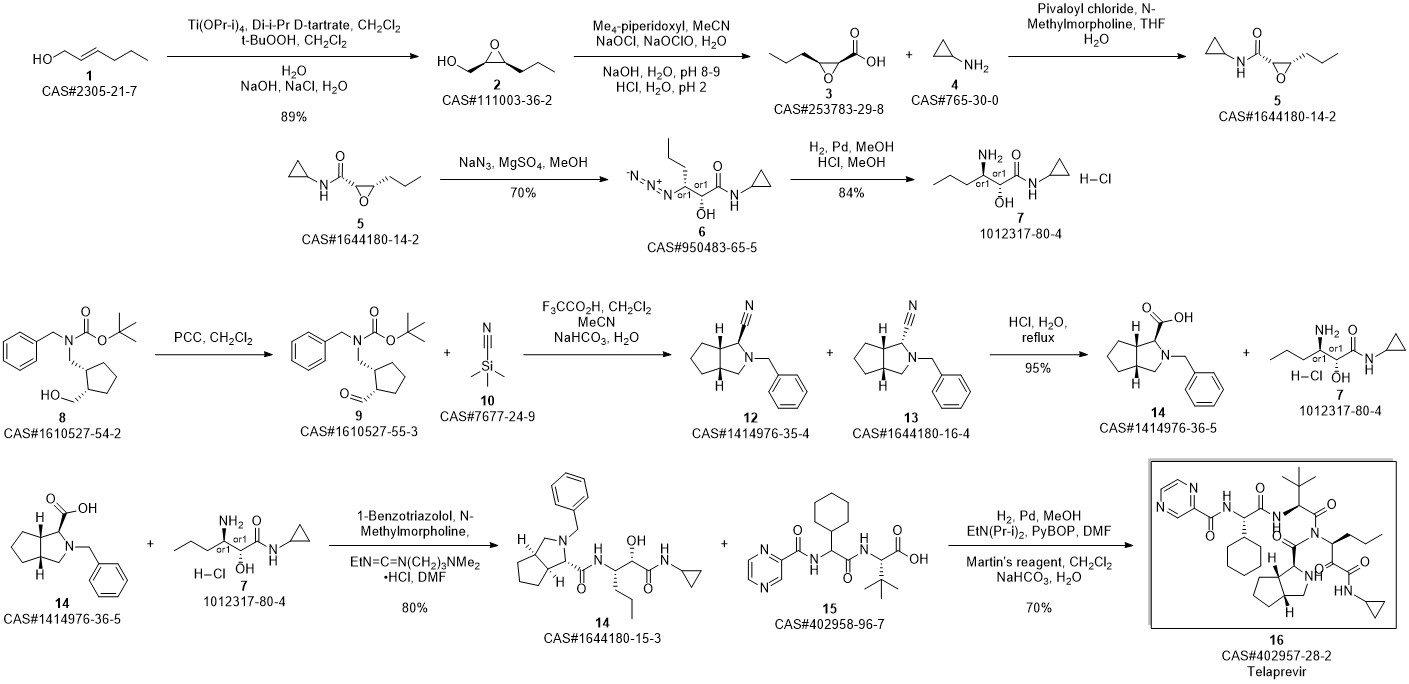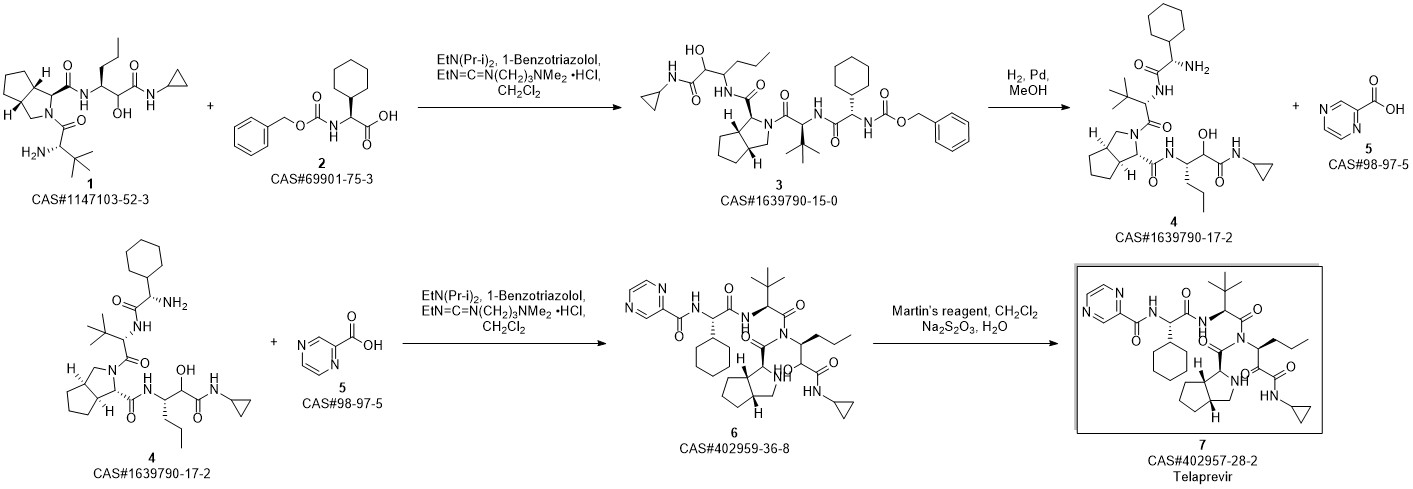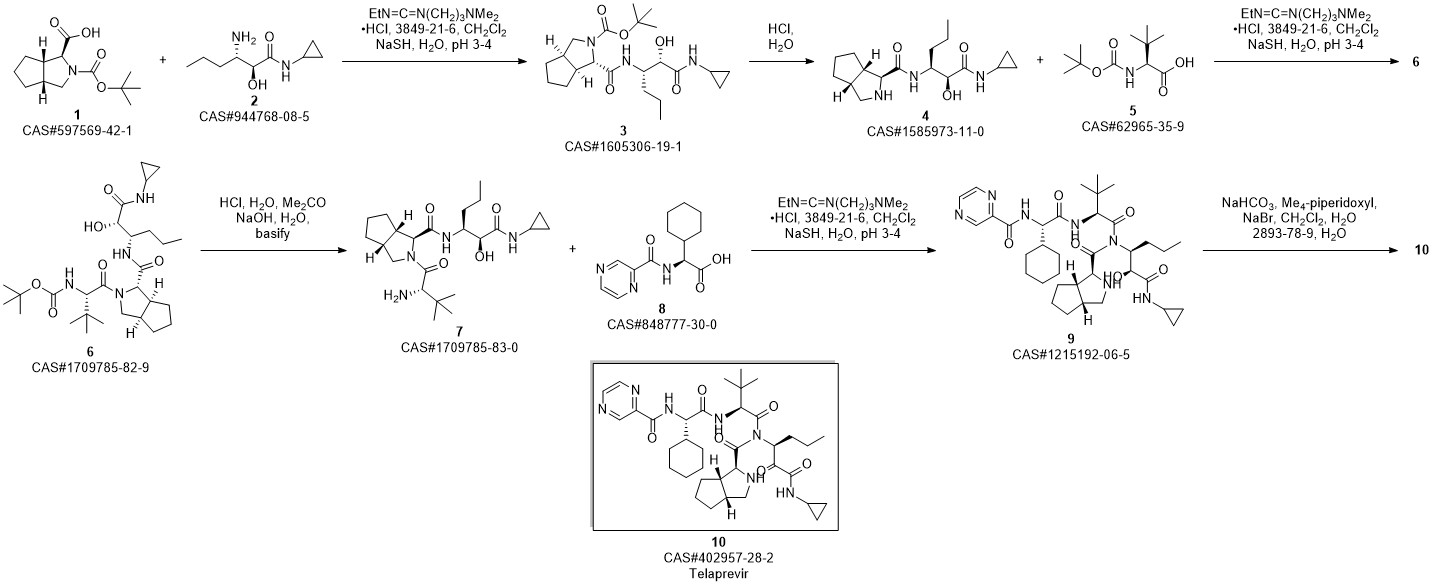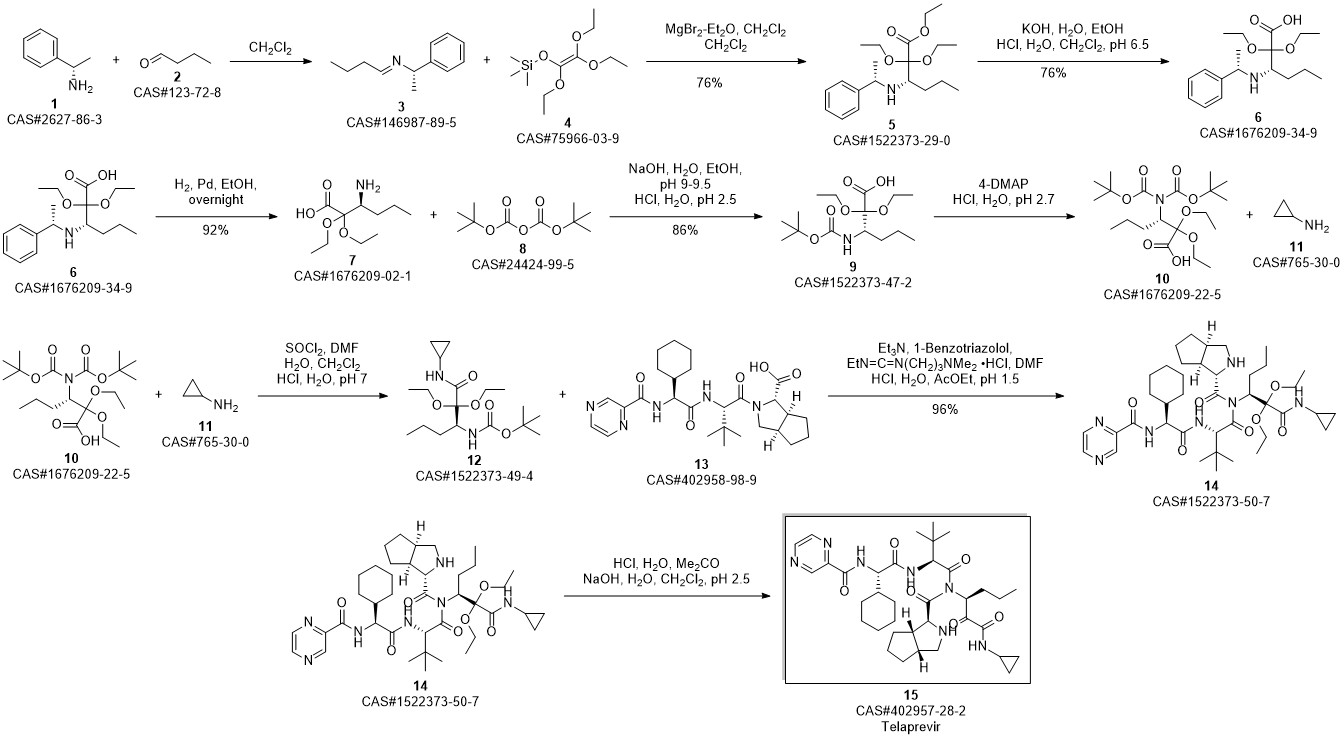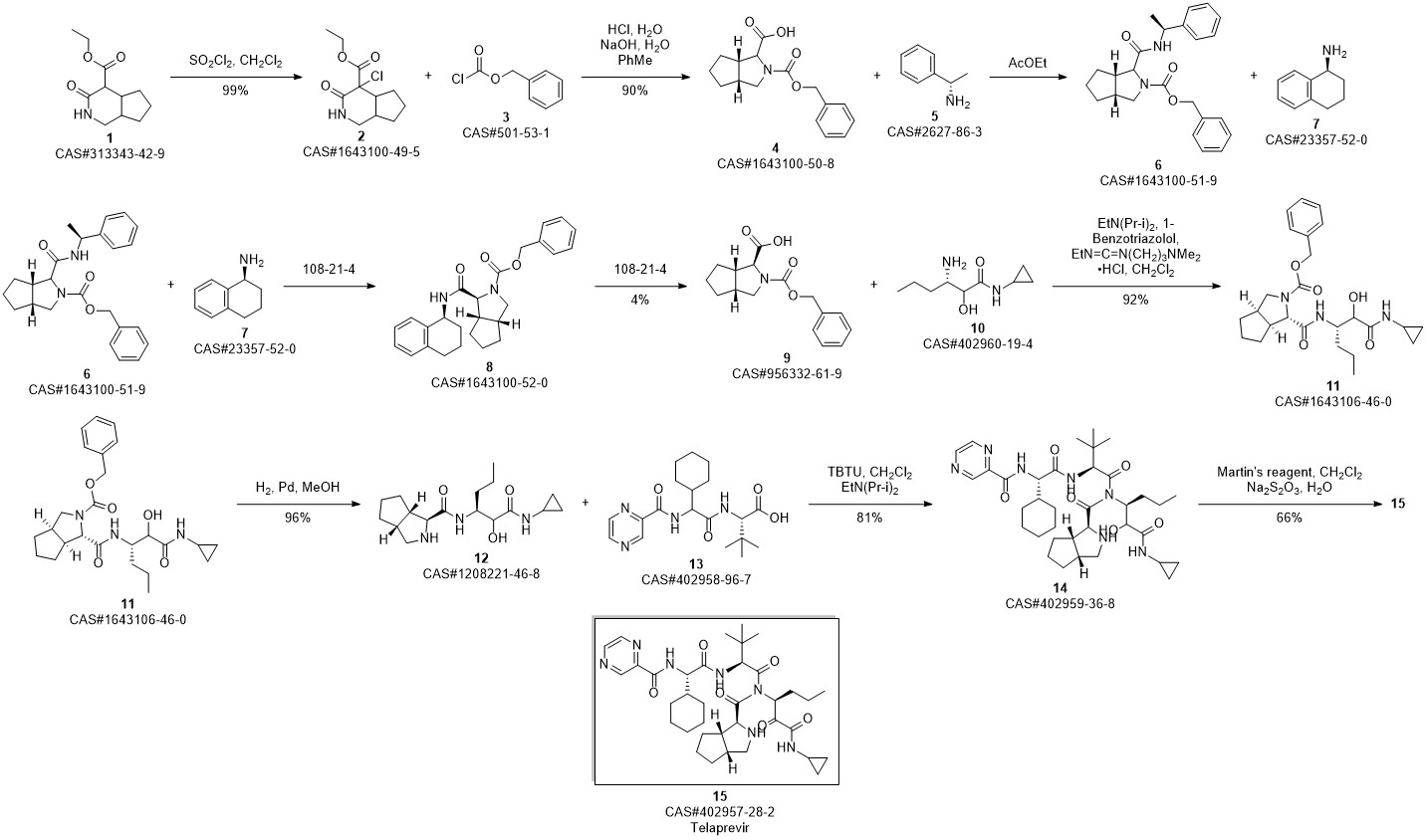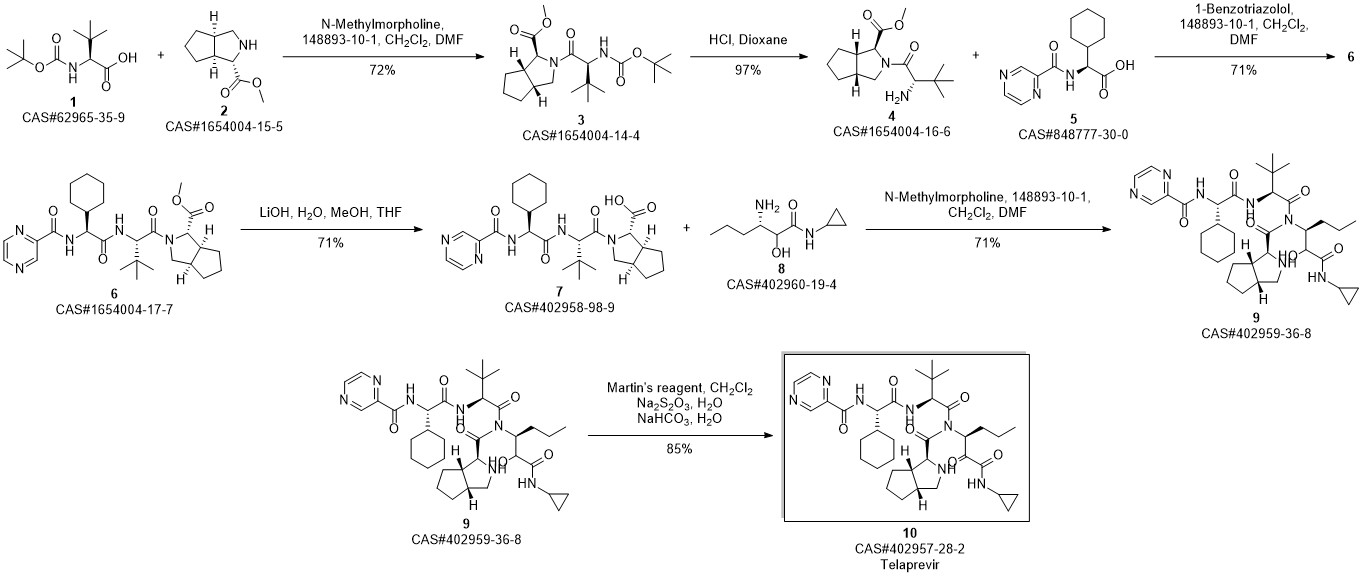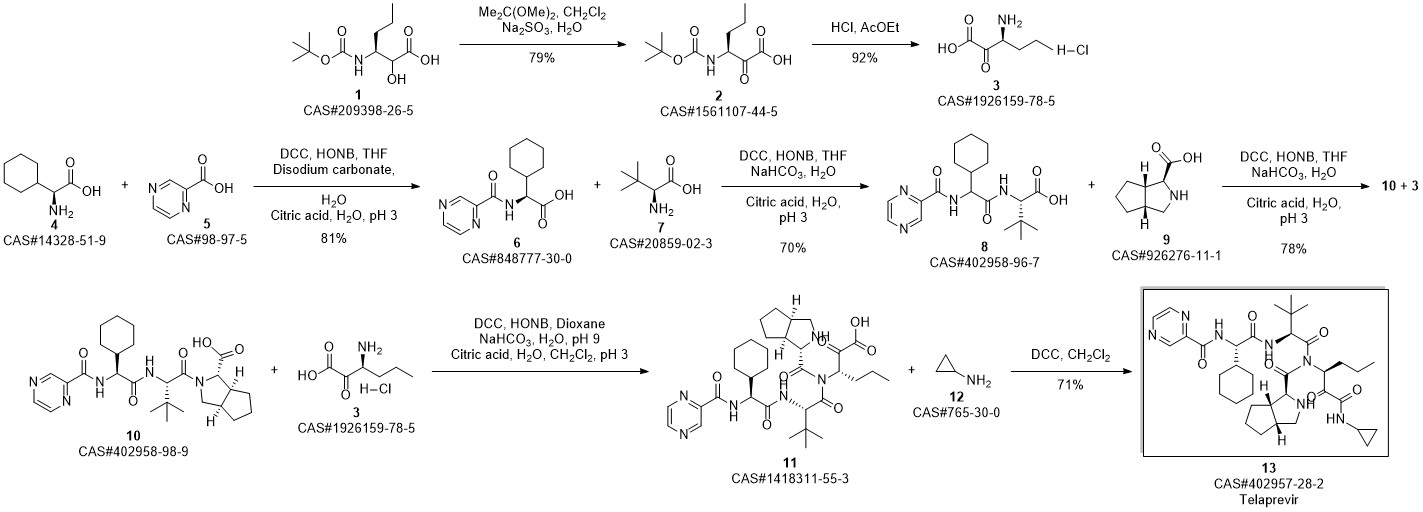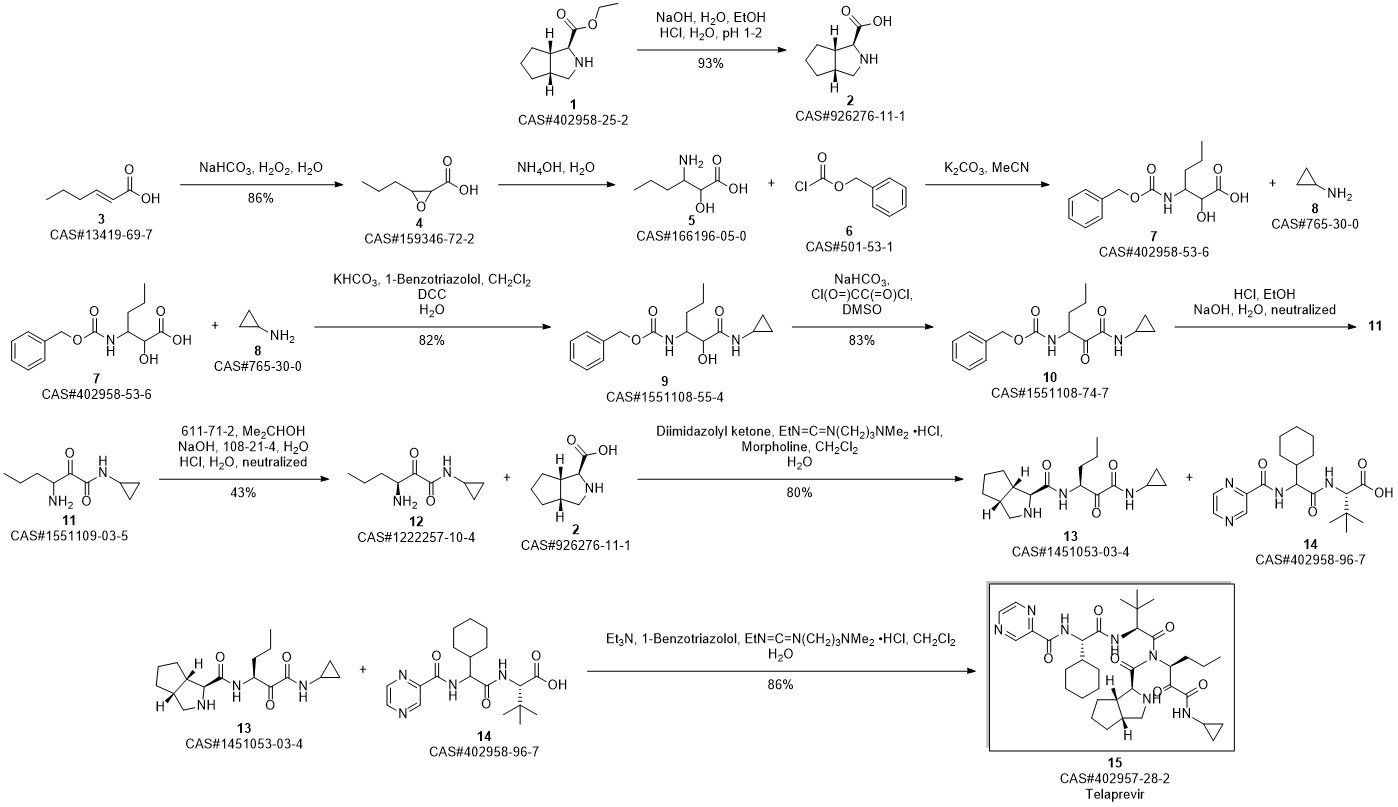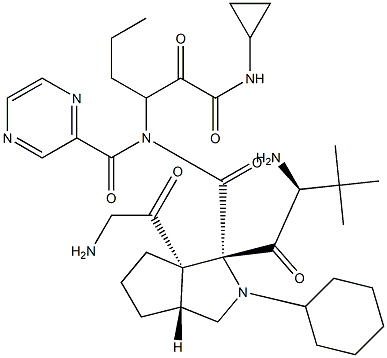
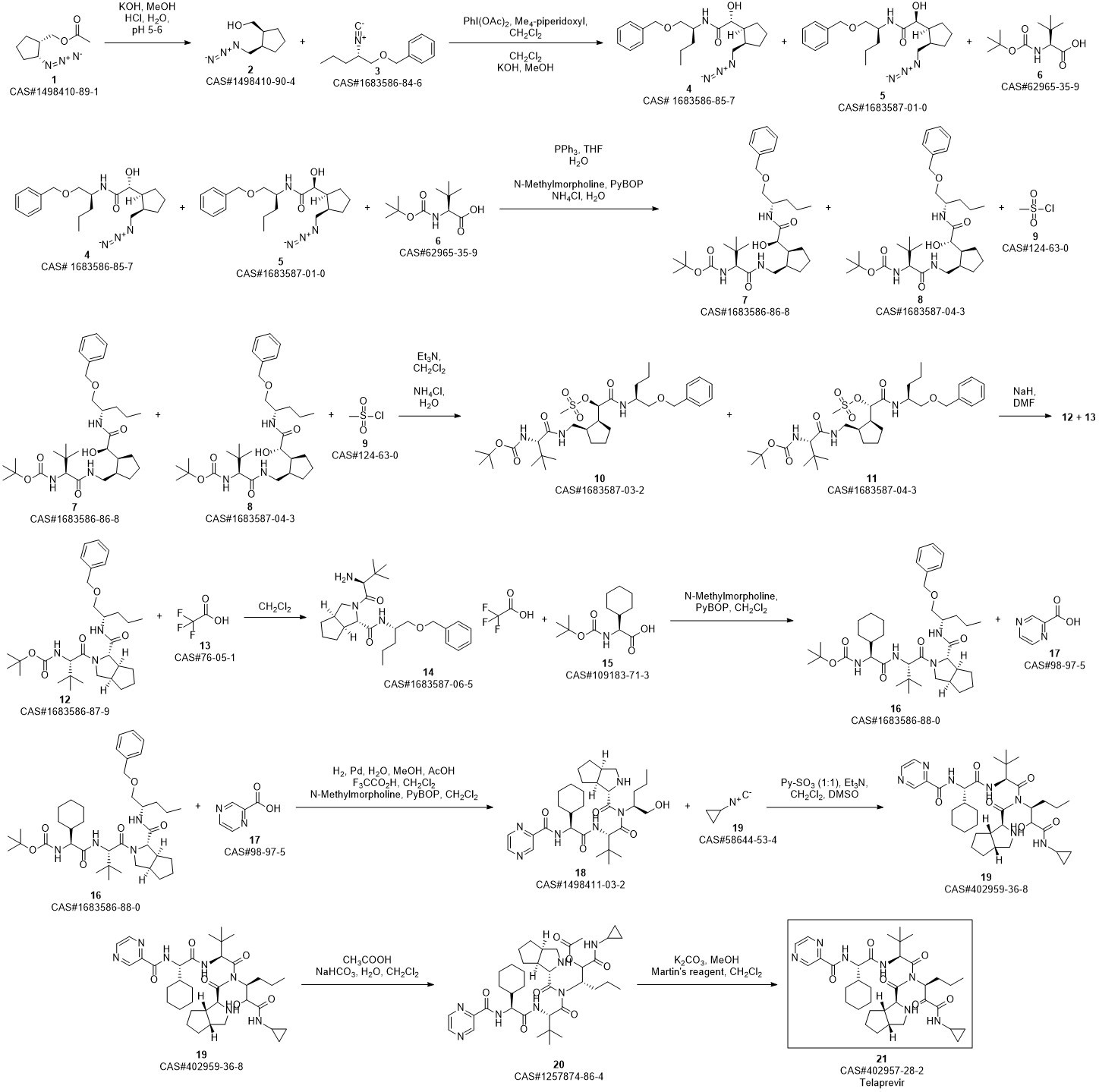
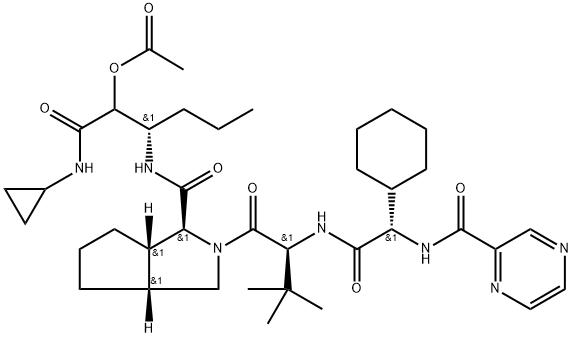
Telaprevir synthesis
- Product Name:Telaprevir
- CAS Number:402957-28-2
- Molecular formula:C36H53N7O6
- Molecular Weight:679.85
Moni, Lisa; Banfi, Luca; Basso, Andrea; Carcone, Luca; Rasparini, Marcello; Riva, Renata. Ugi and Passerini Reactions of Biocatalytically Derived Chiral Aldehydes: Application to the Synthesis of Bicyclic Pyrrolidines and of Antiviral Agent Telaprevir. Journal of Organic Chemistry. Volume 80. Issue 7. Pages 3411-3428. Journal; Online Computer File. (2015).
1257874-86-4
2 suppliers
inquiry

402957-28-2
271 suppliers
$14.00/1mg
Yield:402957-28-2 80%
Reaction Conditions:
Stage #1:C38H57N7O7 with methanol;potassium carbonate at 20; for 0.5 h;
Stage #2: with Dess-Martin periodane in dichloromethane at 20;
Steps:
30
250 μ of saturated K2C03 was added to a solution of 14 (0.514 g, 0.75 mmol) in MeOH (20 ml) at room temperature. The reaction mixture was stirred for 30 minutes at room temperature resulting in a pale yellow suspension. After full conversion (as judged by TLC analysis), the reaction mixture was washed with 20 ml brine, the aqueous layer was washed again with 10 ml CH2C12 (2x). The organic layers were collected, dried with MgS04 and concentrated in vacuo, to yield a pale yellow solid. The yellow solid was dissolved in CH2CI2 (10 ml) and Dess-Martin periodinane (0.650 g, 1.532 mmol) was added at room temperature. The reaction mixture was stirred overnight before adding saturated NaHC03 solution (10 ml) and saturated Na2S203 solution (10 ml). This mixture was stirred for 10 minutes, separated and the aqueous layers were washed with EtOAc (2 x 10 ml). The organic layers were collected, dried with MgSC^ and concentrated in vacuo to give the crude product as an 83: 13:4 mixture of diastereomers. After silica gel flash chromatography (1% MeOH in CH2C12), 1 (0.412 mg, 0.61 mmol, 80%) was obtained as a white solid. lU NMR (500.23 MHz, DMSO-i?: 5 = 9.19 (d, J= 1.4 Hz, 1H), 8.91 (d, J= 24.5 Hz, 1H), 8.76 (dd, J = 1.5, 2.5 Hz, 1H), 8.71 (d, J= 5.3 Hz, 1H), 8.49 (d, J= 9.2 Hz, 1H), 8.25 (d, J = 6.8 Hz, 1H), 8.21 (d, J = 8.9 Hz, 1H), 4.94 (m, 1H), 4.68 (dd, J= 6.5, 9.0 Hz, 1H), 4.53 (d, J = 9.0 Hz, 1H), 4.27 (d, J = 3.5 Hz, 1H), 3.74 (dd, J = 8.0, 10 Hz, 1H), 2.74 (m, 1H), 3.64 (d, J = 3.5 Hz, 1H), 0.92 (s, 9H), 0.87 (t, 3H), 0.84-1.40 (m, 23H), 0.65 (m, 2H), 0.56 (m, 2H); 13C NMR (125.78 MHz, CDC13): δ = 197.0 (C), 171.8 (C), 170.4 (C), 169.0 (C), 162.1 (C), 161.9 (C), 147.9 (CH), 144.0 (C), 143.4 (CH), 56.4 (CH), 56.3 (CH), 54.2 (CH), 53.4 (CH), 42.3 (CH), 41.3 (CH), 32.1 (CH), 31.8 (CH), 31.6 (CH), 29.1 (CH), 28.0 (CH), 26.4 (CH3); (cm4): 3302 (m), 2928 (m), 2858 (w), 1658 (s), 1620 (s), 1561 (s), 1442 (m); HRMS (ESI, 4500 V): m/z calcd. for C36H53N706Na+ ([M + Na]+) 702.3950, found 702.3941.
References:
VERENIGING VOOR CHRISTELIJK HOGER ONDERWIJS, WETENSCHAPPELIJK ONDERZOEK EN PATI?NTENZORG;RUIJTER, Eelco;ORRU, Romano;ZNABET, Anass;POLAK, Marloes;TURNER, Nicholas WO2011/103932, 2011, A1 Location in patent:Page/Page column 50; 51
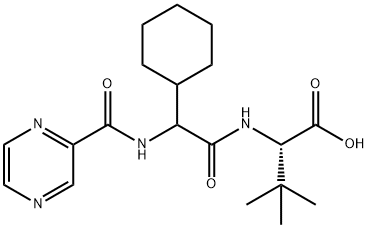
402958-96-7
127 suppliers
$37.00/1g

402957-28-2
271 suppliers
$14.00/1mg