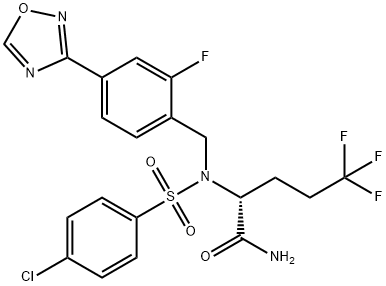
Avagacestat (BMS-708163) synthesis
- Product Name:Avagacestat (BMS-708163)
- CAS Number:1146699-66-2
- Molecular formula:C20H17ClF4N4O4S
- Molecular Weight:520.89
![1,2,4-Oxadiazole, 3-[4-(broMoMethyl)-3-fluorophenyl]-](/CAS/GIF/1146699-64-0.gif)
1146699-64-0
31 suppliers
$190.00/100mg
![PentanaMide, 2-[[(4-chlorophenyl)sulfonyl]aMino]-5,5,5-trifluoro-, (2R)-](/CAS/GIF/1146699-67-3.gif)
1146699-67-3
16 suppliers
inquiry

1146699-66-2
112 suppliers
$35.00/1mg
Yield:1146699-66-2 50%
Reaction Conditions:
with hydroxylamine;tetra-(n-butyl)ammonium iodide;caesium carbonate in acetonitrile at 15 - 35;Product distribution / selectivity;
Steps:
3.B.B.2
Step B, Procedure 2 To a suitable vessel was added (R)-2-(4-chlorophenylsulfonamido)-5,5,5-trifluoropentanamide (2.68 kg, 7.77 mol, 1 eq), 3-(4-(bromomethyl)-3-fluorophenyl)-1,2,4-oxadiazole (2.00 kg, 7.78 mol, 1 eq), cesium carbonate (1.65 kg, 5.06 mol, 0.65 eq), tetrabutylammonium iodide (0.29 kg, 0.78 mol, 0.1 eq) and acetonitrile (12.0 L 4.5 L/kg). The reaction was heated to 35° C. until complete by HPLC (3-(4-(bromomethyl)-3-fluorophenyl)-1,2,4-oxadiazole <0.5 relative AP by HPLC). The reaction was cooled to 15° C. and water (10.72 L, 4 L/kg) was added with stirring followed by glacial acetic acid (0.22 kg) to bring the pH of the reaction to <6.5. The stirring was stopped and the phases were separated (the top layer contained the product). To the product rich layer was added toluene (26.8 kg, 31 L, 10 kg/kg) followed by brine solution (20% w/w, 6.39 kg, 2 L/kg) and the layers were separated (the top layer contained the product). The mixture was distilled at ~50° C. under vacuum (200 mbar) until acetonitrile was removed. The concentration was adjusted with additional toluene if needed after distillation to ensure total volume in the reactor was ~10 L/kg. Isopropyl alcohol (0.48 kg, 0.2 L/kg) was charged and the batch was cooled to 15° C. to initiate crystallization. The resulting slurry was filtered and washed with cold toluene (18.65 kg, 21.56 L, 8 L/kg). The crude cake was tray dried under vacuum at 50° C. until loss on drying was <1.0%. The dry cake was added to a 100 L reactor along with isopropyl alcohol (27.34 kg, 34.8 L, 13 L/kg) and hydroxylamine (50% aqueous solution, 0.05 kg, 1.51 mol, 0.2 eq). The mixture was heated to 65° C. and monitored by HPLC until (R)-2-(4-chloro-N-(4-cyano-2-fluorobenzyl)phenylsulfonamido)-5,5,5-trifluoropentanamide was <0.4 AP. The reaction was then distilled (pot temperature ~50° C., vacuum 300 mbar) until reaction volume was ~60% of original. Acetonitrile (5.36 kg, 2 L/kg) was charged and the reaction temperature was increased to 70° C. to achieve complete dissolution. Water (11.26 L, 4.2 L/kg) was charged slowly while keeping the reaction temperature >65° C. The reaction was cooled to 15° C. over 2 hours and crystallization occurred. The slurry was filtered and washed with cold aqueous isopropyl alcohol (2:1 IPA:water by volume). The cake was dried in a vacuum oven until loss on drying was <1%. The product was then recrystallized by dissolving in acetonitrile (2 L/kg based on weight of input of dried cake) and methanol (6 L/kg) and then heated to 50° C. Water (4 L/kg) was added slowly, keeping the reaction temperature >50° C. The reaction was cooled to 15° C. over 2 hours. The resulting slurry was filtered and washed with a solution of methanol:acetonitrile:water (6:2:4, 5 L/kg) to give (2R)-2-[[(4-chlorophenyl)sulfonyl][[2-fluoro-4-(1,2,4-oxadiazol-3-yl)phenyl]methyl]amino]-5,5,5-trifluoropentanamide (2.02 kg, 50% yield) as a white solid.
References:
Bristol-Myers Squibb Company US2009/111858, 2009, A1 Location in patent:Page/Page column 21-22