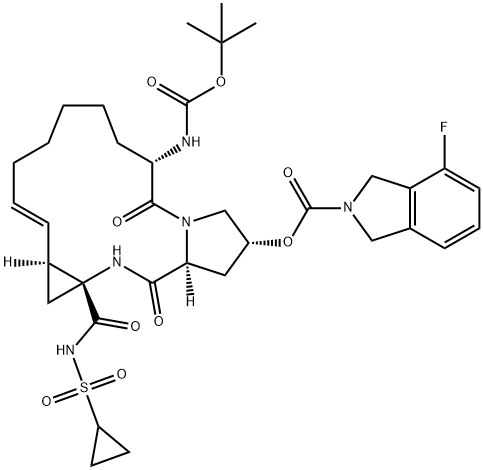
Danoprevir synthesis
- Product Name:Danoprevir
- CAS Number:850876-88-9
- Molecular formula:C35H46FN5O9S
- Molecular Weight:731.83
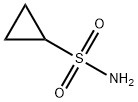
154350-29-5
284 suppliers
$6.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

850876-88-9
109 suppliers
$49.00/1mg
Yield:-
Reaction Conditions:
Stage #1:6-[[(tert-butoxy)carbonyl]amino]-2-[[(4-fluoro-1,3-dihydro-2H-isoindol-2-yl)carbonyl]oxy]-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-hexadecahydro-5,16-dioxo-(2R,6S,12Z,13aS,14aR,16aS)-cyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5H)-carboxylic acid with acetic anhydride;sodium carbonate in tetrahydrofuran at 45; for 8.5 h;
Stage #2:cyclopropanesulphonamide with potassium carbonate in tetrahydrofuran at 62; for 17 h;
Steps:
22
To a suspension of 30.0 g (0.043 mol) of carboxylic acid (product of example 20 with an assay of 90.2%(m/m)) and 14.0 g of sodium carbonate in 225 g of tetrahydrofuran was added at 45°C within 30 minutes 7.60 g (0.074 mol) of acetic acid anhydride and the resulting mixture was stirred at 45°C for 8 hours. To the resulting suspension was then added 30.2 g (0.17mol) of potassium carbonate and 8.0 g (0.065 mol) of cyclopropyl sulfonamide. The mixture was heated to 62°C and stirred at this temperature for 17 hours. The mixture was concentrated to a residual volume of 200 ml and then treated with 200 g of water. The biphasic mixture was stirred for 15 minutes and the layers were then allowed to separate. The lower aqueous phase was removed. The organic phase was diluted with 90 g of ethyl acetate and washed with 3% sulfuric acid (1x140 g) and water (3x130 g). The organic layer was concentrated to dryness and then diluted with 400 ml of ethyl acetate. Residual amounts of water were removed by a continuous azeotropic distillation with ethyl acetate. The mixture was then treated at 100C with 20 ml of methanol, followed by 10.0 g of sodium methylate (30% in methanol). From the resulting
References:
F. HOFFMANN-LA ROCHE AG WO2009/124853, 2009, A1 Location in patent:Page/Page column 53-54

154350-29-5
284 suppliers
$6.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

850876-88-9
109 suppliers
$49.00/1mg