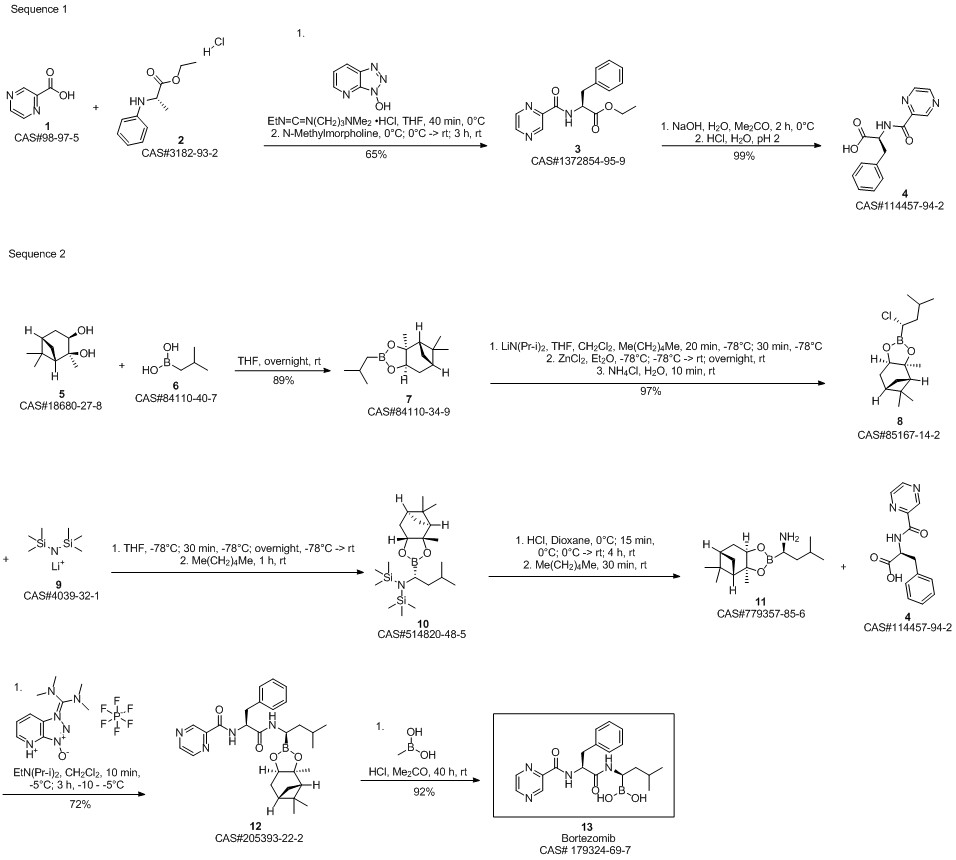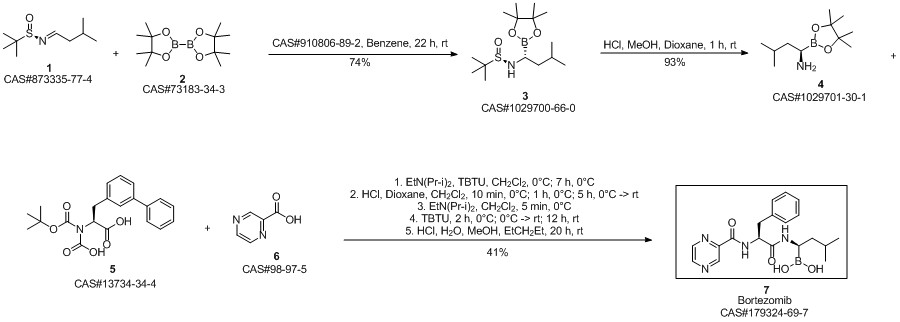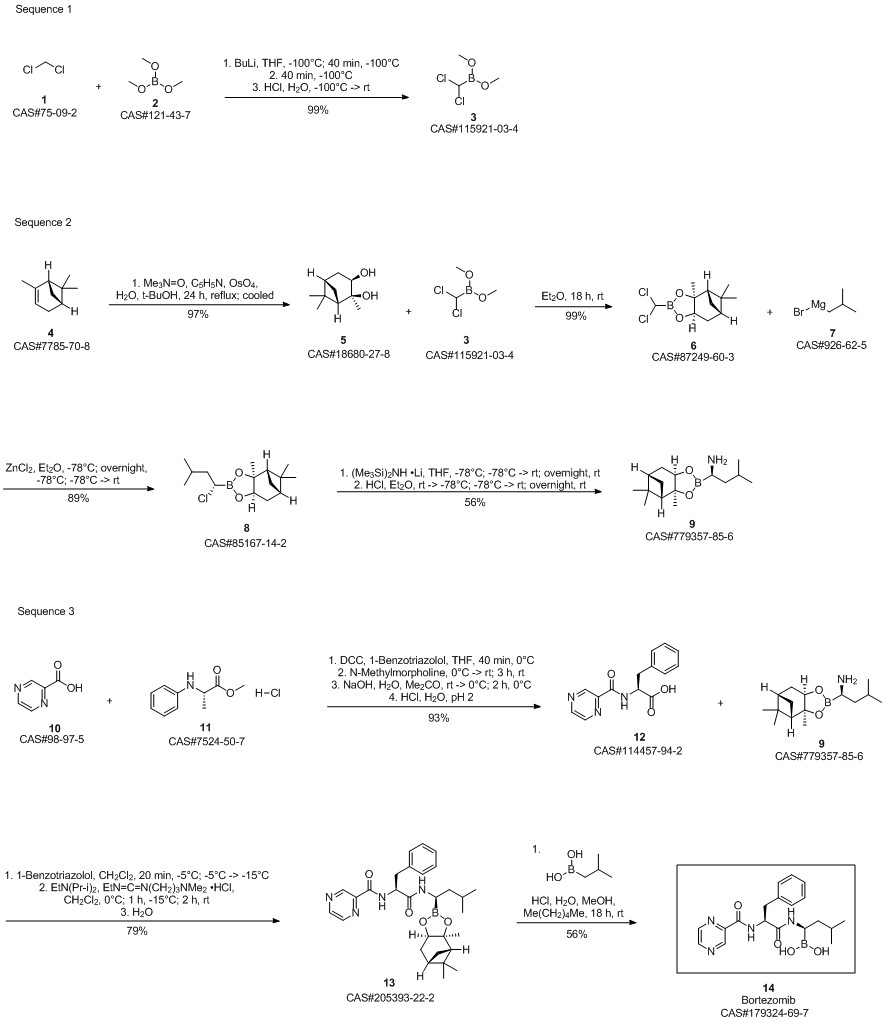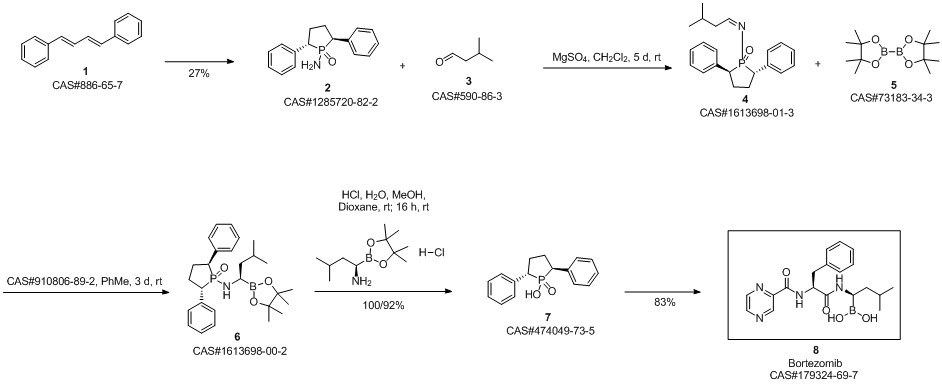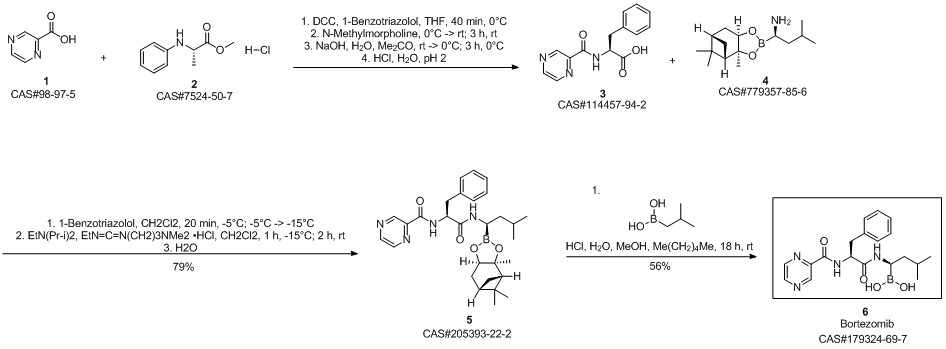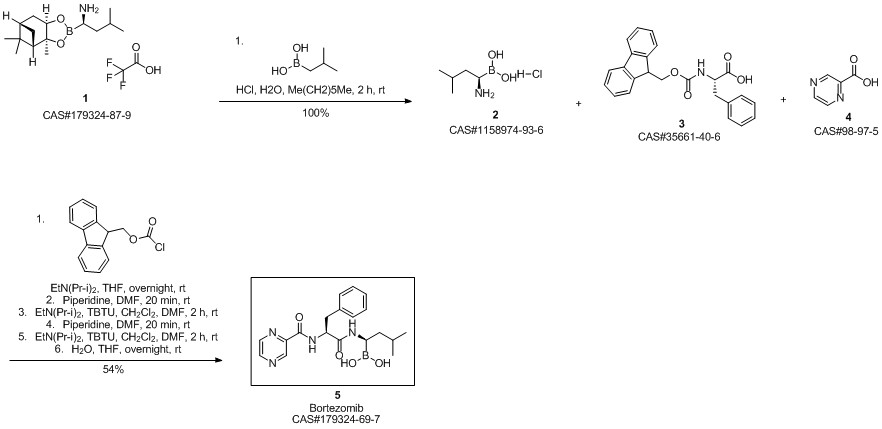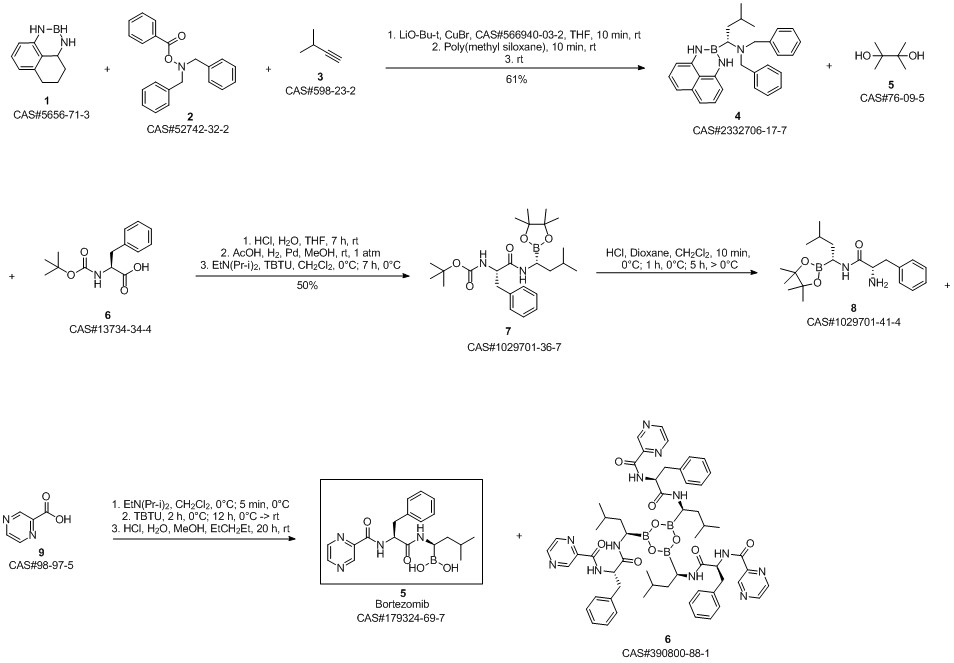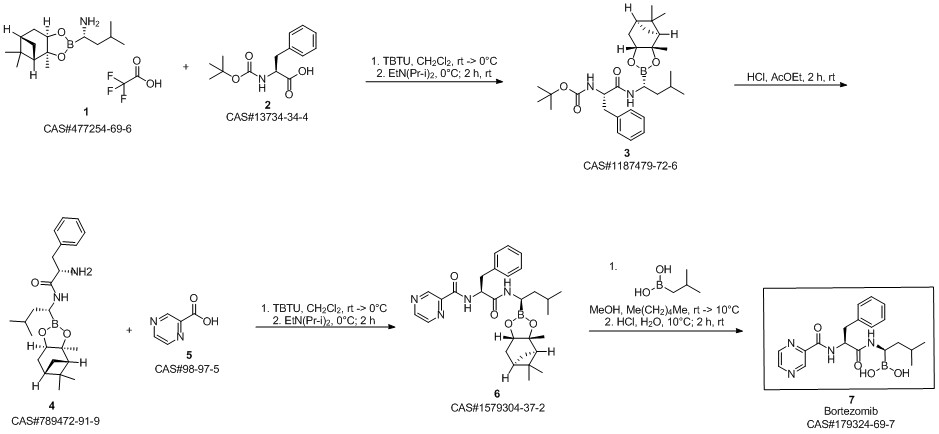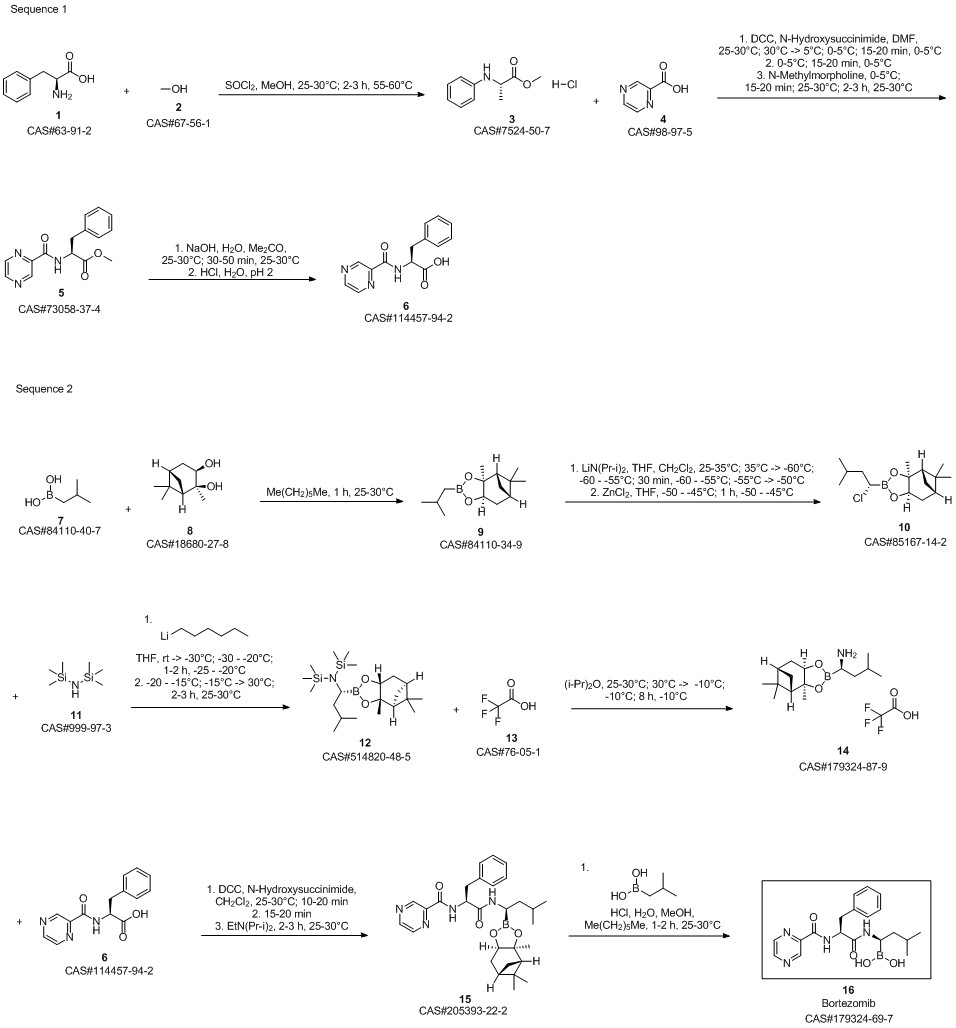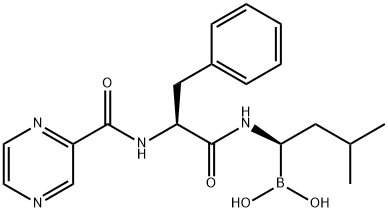
Bortezomib synthesis
- Product Name:Bortezomib
- CAS Number:179324-69-7
- Molecular formula:C19H25BN4O4
- Molecular Weight:384.24
Peuchmaur, Marine; Lacour, Marie-Agnes; Sevalle, Jean; Lisowski, Vincent; Touati-Jallabe, Youness; Rodier, Fabien; Martinez, Jean; Checler, Frederic; Hernandez, Jean-Francois. Further characterization of a putative serine protease contributing to the γ-secretase cleavage of β-amyloid precursor protein. Bioorganic & Medicinal Chemistry. Institut des Biomolecules Max Mousseron, UMR5247 CNRS, Faculte de Pharmacie. Universites Montpellier 1 and 2. Montpellier, Fr. 34093. Volume 21. Issue 4. Pages 1018-1029. 2013
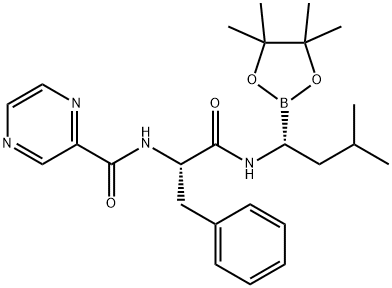
1029701-48-1
1 suppliers
inquiry
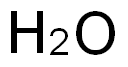
7732-18-5
469 suppliers
$13.50/100ML

179324-69-7
707 suppliers
$16.00/5mg
Yield:179324-69-7 390 g
Reaction Conditions:
with hydrogenchloride;Dihydroxy-isobutyl-boran in methanol;hexane at 15 - 20; for 1 h;
Steps:
13
Dissolve the spin-dried b8 with methanol (4.75Kg), add it to the 30L reactor, then add n-hexane (3.95Kg) and isobutylboronic acid (0.3Kg), the temperature in the reactor was adjusted to 15-20 ° C, 1N hydrochloric acid (3Kg) was added dropwise, and the addition was completed in about 20-30 minutes. After 1 hour of reaction, the content of b8 was less than 1% by HPLC, and the reaction was terminated. Aspirate the reaction liquid into a 50L liquid separator to separate the n-hexane solution,Add an equal amount of n-hexane for extraction once. Then add pure water (12Kg) and dichloromethane (10Kg) to extract the product, separate the dichloromethane solution, add dichloromethane (5Kg) to extract, combine the extracts, and wash twice with water. Spin dry at 33 ° C, add ethyl acetate (5.52Kg) to dissolve, let stand at room temperature for 2 hours, place in freezer overnight, filter the next day, wash once with ethyl acetate, pump to dry, leave room for 2 hours, vacuum dry at 30 ° C After 10 hours, crude bortezomib (390g) was obtained, the yield in two steps was 67.66%, and the chromatographic purity 99.4%, melting point 169-173 ° C,
References:
CN104860975,2019,B Location in patent:Paragraph 0114; 0116

1029701-48-1
1 suppliers
inquiry

179324-69-7
707 suppliers
$16.00/5mg
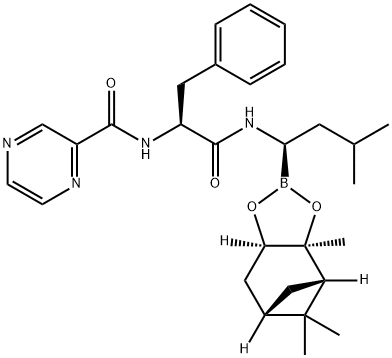
205393-22-2
113 suppliers
inquiry

179324-69-7
707 suppliers
$16.00/5mg
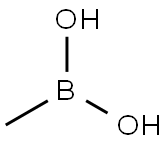
13061-96-6
298 suppliers
$6.00/1g

1029701-48-1
1 suppliers
inquiry

179324-69-7
707 suppliers
$16.00/5mg

205393-22-2
113 suppliers
inquiry

13061-96-6
298 suppliers
$6.00/1g

179324-69-7
707 suppliers
$16.00/5mg