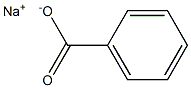
Sodium benzoate synthesis
- Product Name:Sodium benzoate
- CAS Number:532-32-1
- Molecular formula:C7H5NaO2
- Molecular Weight:144.1032
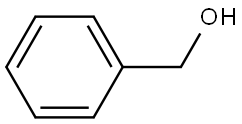
100-51-6
1439 suppliers
$5.00/100g

532-32-1
1338 suppliers
$5.00/25g
Yield:532-32-1 92%
Reaction Conditions:
with oxygen;sodium hydroxide in neat (no solvent) at 179.84; for 8 h;Reagent/catalyst;
Steps:
3.3. Catalytic oxidation of benzyl alcohol
The catalytic oxidation of benzyl alcohol to sodium benzoateunder solvent-free conditions in the presence of NaOH isknown as the Cannizzaro reaction. Benzyl alcohol was oxidizedto benzoic aldehyde that instantaneously transformed intosodium benzoate in an alkaline environment.Table 4 shows the catalytic results using monometallic andbimetallic catalysts towards the oxidation of benzyl alcohol to sodium benzoate and benzoic acid under solvent-free conditions.Because the desired product was of high purity (selectivityof 100%), the yield was used to evaluate the catalytic activity.As observed, the yields obtained in the presence of themonometallic Au/CeO2 and Pd/CeO2 catalysts are relativelylow. Following addition of the second metal, distinctly improvedcatalytic activity was observed. The catalyst with anAu/Pd ratio of 3/1 displayed the best catalytic performance,with a corresponding yield of 92% achieved after 8 h of reaction.In contrast with Pd/CeO2, the yields generated in thepresence of the bimetallic catalysts decreased with increasingPd amounts. The space time yield (STY) obtained in the presenceof the AuPd/CeO2 catalysts was also compared, and theresults are shown in Table 4. The STY values markedly increasedupon introduction of Pd into the monometallicAu/CeO2 catalyst and reached maxima at an Au/Pd molar ratioof 3/1 (i.e., STY 2.81 h-1), then decreased with further increasein the loading amount of Pd. This indicates the existence of asuitable Au/Pd molar ratio required to generate optimal catalytic activity.To examine the stability of the bimetallic catalysts, cyclingexperiments were conducted using the 3Au1Pd/CeO2 catalyst,and the results are shown in Fig. 8. The bimetallic catalyst waseasily recovered and reused for more than seven successivereactions without significant loss in the catalytic activity, indicatingthe high stability of the as-prepared AuPd/CeO2 catalyst.Furthermore, the activity of the AuPd/CeO2 catalyst is higherthan that of previously studied AuAg/TiO2 (10 h, yield 82%)[37]. Moreover, the use of AuPd/CeO2 catalyst requires loweramounts of catalyst and milder reaction conditions including alower reaction temperature and a shorter reaction time.Therefore, the as-prepared AuPd/CeO2 is an excellent catalystcandidate for the oxidation of benzyl alcohol to benzoic acid.
References:
Zhang, Zhaoyan;Wang, Ying;Li, Xian;Dai, Wei-Lin [Chinese Journal of Catalysis,2014,vol. 35,# 11,p. 1846 - 1857]
3686-66-6
56 suppliers
$40.00/500mg

532-32-1
1338 suppliers
$5.00/25g
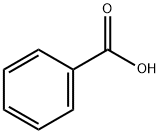
65-85-0
1413 suppliers
$10.00/25g

532-32-1
1338 suppliers
$5.00/25g