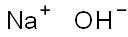
Sodium hydroxide synthesis
- Product Name:Sodium hydroxide
- CAS Number:1310-73-2
- Molecular formula:NaOH
- Molecular Weight:39.99711
Sodium hydroxide is manufactured together with chlorine by electrolysis of sodium chloride solution. Various types of electrolytic cells are used commercially. They include the mercury cell, the diaphragm cell, and the membrane cell.
A saturated solution of brine is electrolyzed. Chlorine gas is liberated at the anode and sodium ion at the cathode. Decomposition of water produces hydrogen and hydroxide ions. The hydroxide ion combines with sodium ion forming NaOH. The overall electrolytic reactions may be represented as:
2Na+ + 2Cl-+ 2H2O → Cl2 (g) + H2 (g) + 2NaOH (aq)
The mercury cell proceeds in two stages that occur separately in two cells. The first is known as the brine cell or the primary electrolyzer in which sodium ion deposits on the mercury cathode forming amalgam, while chlorine gas is liberated at the anode:
Na+ + Cl–→ Na-Hg (cathode) + ½Cl2(g) (anode)
In the second cell, known as the decomposer cell, a graphite cathode is used while sodium amalgam serves as the anode. Water reacts with the sodium metal of the amalgam in the decomposer:
Na-Hg + H2O → Na+ + OH– + ½H2↑ + Hg
In chlor-alkali diaphragm cells, a diaphragm is employed to separate chlorine liberated at the anode from the sodium hydroxide and hydrogen generated at the cathode. Without a diaphragm, the sodium hydroxide formed will combine with chlorine to form sodium hypochlorite and chlorate. In many cells, asbestos diaphragms are used for such separation. Many types of diaphragm cells are available.
Sodium hydroxide is produced either as an anhydrous solid or as a 50% aqueous solution.
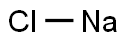
7647-14-5
1144 suppliers
$5.00/500g
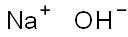
1310-73-2
1374 suppliers
$14.00/25g
Yield:1310-73-2 90%
Reaction Conditions:
with clay;sodium carbonate in water;byproducts: NaAlO2; reaction of bricks of NaCl and clay with hot gases and overheated vapor in a shaft furnace; melting residue (acidic sodium silicon aluminate) with Na2CO3; product leached with water;; NaOH contains some Na2CO3 and NaAlO2;;
References:
[Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Na: MVol., 43.4, page 175 - 177] XX11492,1887 XX10202,1891 Lunge, G. [Handbuch der Soda-Industrie und ihrer Nebenzweige, 3. Aufl., Friedr. Vieweg und Sohn, Braunschweig 1909, Bd. 3, S. 214]
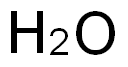
7732-18-5
469 suppliers
$13.50/100ML
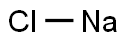
7647-14-5
1144 suppliers
$5.00/500g
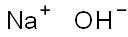
1310-73-2
1374 suppliers
$14.00/25g
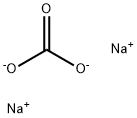
497-19-8
1392 suppliers
$14.00/1KG
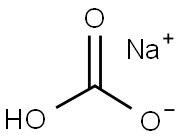
144-55-8
1314 suppliers
$10.00/25g
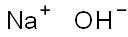
1310-73-2
1374 suppliers
$14.00/25g
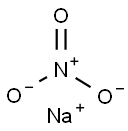
7631-99-4
10 suppliers
$10.00/5g
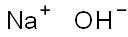
1310-73-2
1374 suppliers
$14.00/25g
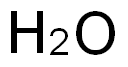
7732-18-5
469 suppliers
$13.50/100ML

497-19-8
1392 suppliers
$14.00/1KG
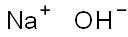
1310-73-2
1374 suppliers
$14.00/25g