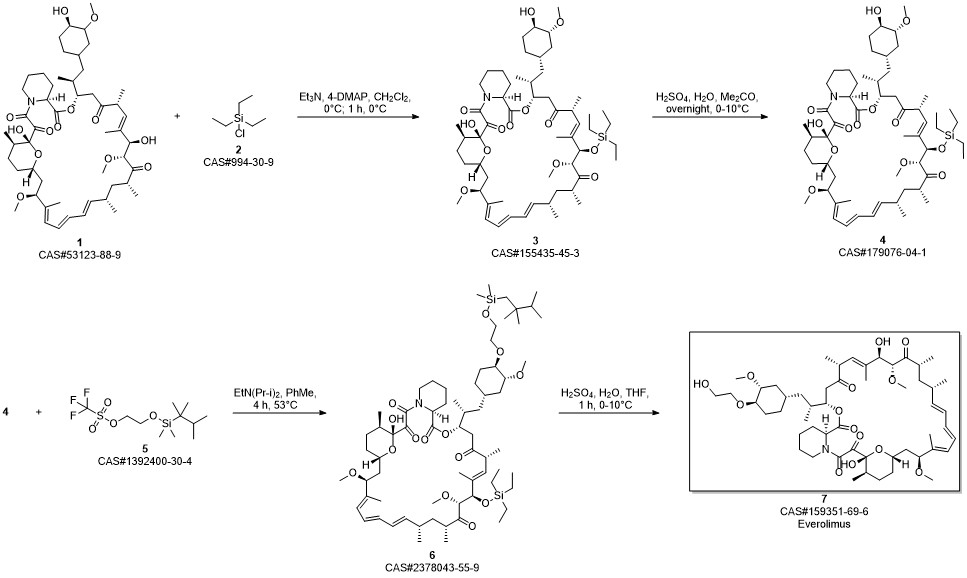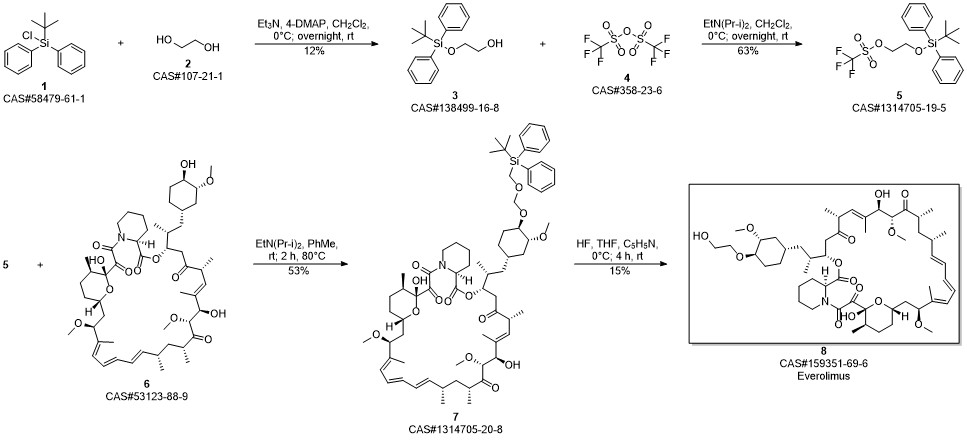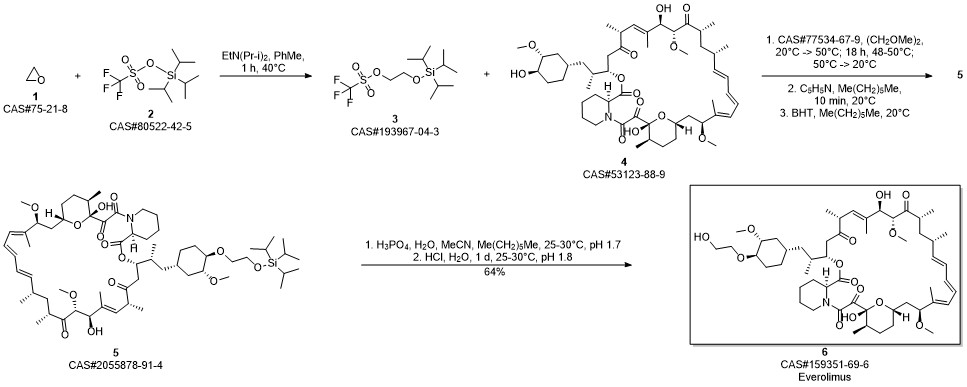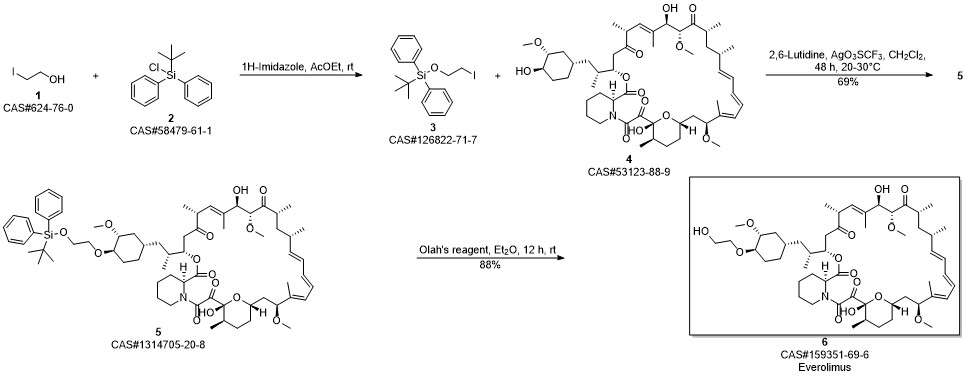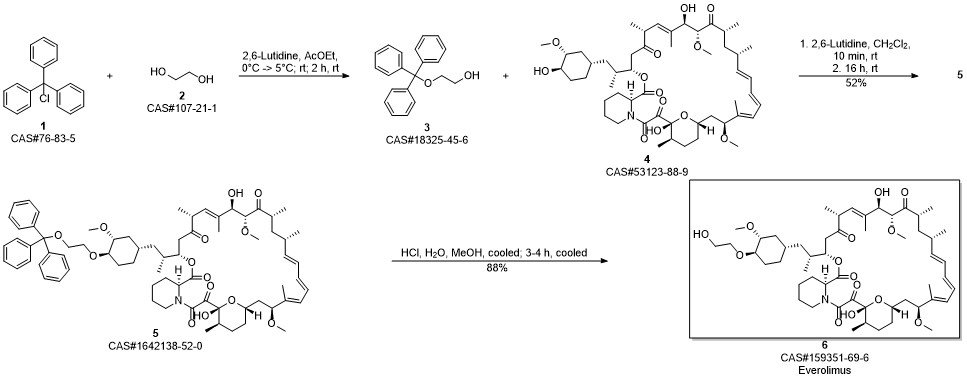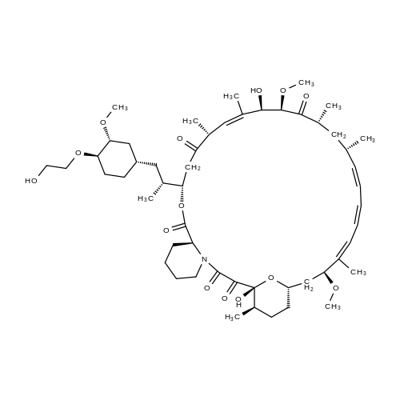
Everolimus synthesis
- Product Name:Everolimus
- CAS Number:159351-69-6
- Molecular formula:C53H83NO14
- Molecular Weight:958.22
Polymer compositions containing a macrocyclic triene compound; Shulze, John E.; Betts, Ronald E.; Savage, Douglas R.; Assignee Sun Bow Co., Ltd., Bermuda; Sun Biomedical Ltd. 2003; Patent Information; Nov 06, 2003; WO 2003090684 A2
![40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin](/CAS/20211123/GIF/159351-68-5.gif)
159351-68-5
18 suppliers
inquiry

159351-69-6
522 suppliers
$8.00/1mg
Yield:159351-69-6 98.1%
Reaction Conditions:
with hydrogenchloride in lithium hydroxide monohydrate;ethyl acetate at 0 - 5;Temperature;
Steps:
19-22 Example 19
At room temperature, compound V (9.64 g, 9 mmol) was added to 50 mL of ethyl acetate, stirred and dissolved, cooled to 05° C., and hydrochloric acid solution (6.75 mL, 6.75 mmol, 1.0 mol/L) was added dropwise, and stirred until the reaction was completed, 50 mL of saturated NaHCO solution was added to the reaction solution, and the solution was separated. The aqueous phase was extracted with 25 mL of ethyl acetate. The organic phases were combined. The organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain everolimus. The crude product, the crude everolimus was dissolved in 20 mL of ethyl acetate, then 20 mL of anhydrous ether was added, stirred for 1 h, and then concentrated under reduced pressure until a solid was precipitated. The obtained solid was added to 20 mL of anhydrous ether, stirred for 30 min, and concentrated to dryness under reduced pressure. Everolimus was obtained, the yield was 98.1%, and the HPLC purity was 99.83%.
References:
CN114539288,2022,A Location in patent:Paragraph 0017; 0096-0106
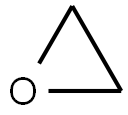
75-21-8
237 suppliers
$39.10/1mL
53123-88-9
794 suppliers
$9.00/10mg

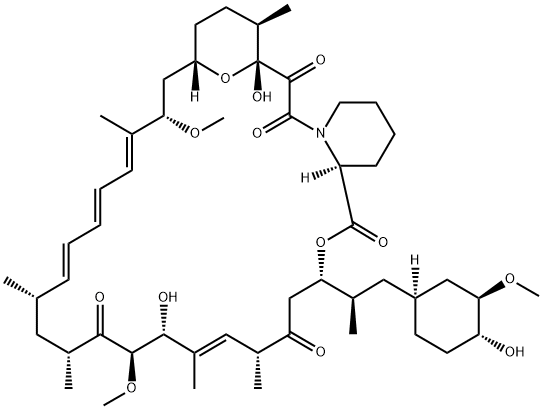
159351-69-6
522 suppliers
$8.00/1mg
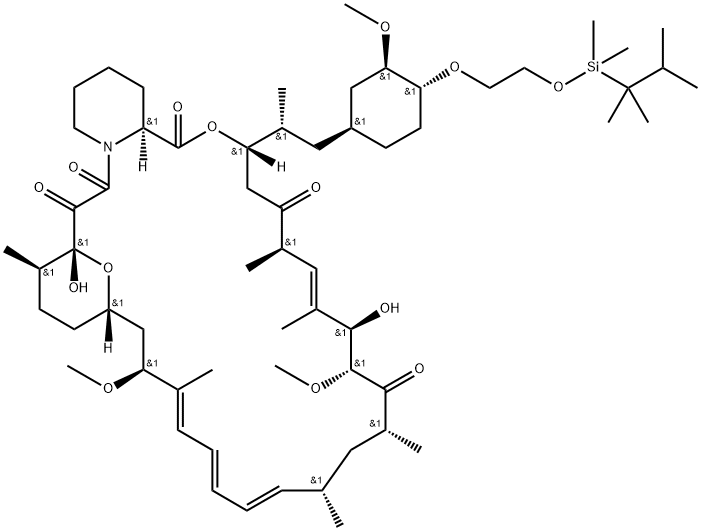
1392400-31-5
2 suppliers
inquiry

159351-69-6
522 suppliers
$8.00/1mg