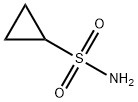
Cyclopropanesulfonamide synthesis
- Product Name:Cyclopropanesulfonamide
- CAS Number:154350-29-5
- Molecular formula:C3H7NO2S
- Molecular Weight:121.16
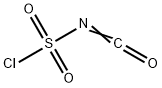
1189-71-5
341 suppliers
$18.00/5g
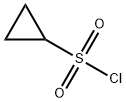
139631-62-2
249 suppliers
$10.00/1g
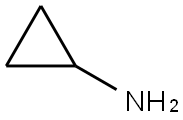
765-30-0
485 suppliers
$10.00/25g

154350-29-5
284 suppliers
$6.00/1g
Yield:154350-29-5 750 mg (6.19 mmol, 87%)
Reaction Conditions:
with ammonia;triethylamine;trifluoroacetic acid in dichloromethane;tert-butyl alcohol;
Steps:
V Preparation of Sulfonyl Compounds
Preparation of Sulfonyl Compounds Compounds S1, S2, S3, and S4, shown above, were prepared according to procedures described in WO 2005/095403 and PCT/US2005/010494, hereby incorporated by references by their entireties. Specifically, to a solution of chlorosulfonylisocyanate (10 mL, 115 mmol) in CH2Cl2 (200 mL) at 0° C. was added t-BuOH (11 mL, 1 eq.). The mixture was stirred for 60 minutes, then added via cannula into a solution of cyclopropylamine (6.6 g) in CH2Cl2 (200 mL) with triethylamine (30 mL) at 0° C. concurrently with a solution of triethylamine (50 mL) in CH2Cl2 (100 mL) via addition funnel. Internal temperature was maintained below 8° C. Stirred at room temperature after completion of addition for 4 hrs. The reaction was then diluted with CH2Cl2 and transferred to a separatory funnel, washed with 1 N HCl (twice, 400 mL each), brine (300 mL), dried (MgSO4), filtered and concentrated. The product was recrystallized from ethyl acetate/hexanes to yield 16.8 g (71.3 mmol, 62%) of S3. Compound S3 was deprotected with trifluoroacetic acid in CH2Cl2 to give compound S4 in quantitative yield. Ammonia gas was bubbled through a gas dispersion tube into THF (40 mL) cooled to 0° C. for 5 minutes. To this solution at 0° C. was added cyclopropylsulfonylchloride (1 gram, 7.1 mmol). The reaction was stirred at room temperature overnight, then filtered through a plug of silica gel, followed by elution with EtOAc to yield 750 mg (6.19 mmol, 87%) of cyclopropylsulfonamide. 1H-NMR (500 MHz, Methanol-d4): 4.79 (s, 2H), 2.59-2.54 (m, 1H), 1.06-0.96 (m, 4H).
References:
US2010/272681,2010,A1

139631-62-2
249 suppliers
$10.00/1g

154350-29-5
284 suppliers
$6.00/1g
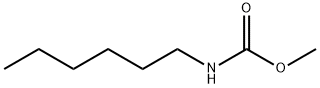
22139-32-8
0 suppliers
inquiry

154350-29-5
284 suppliers
$6.00/1g
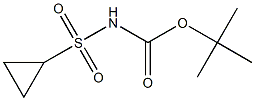
681808-26-4
9 suppliers
inquiry

154350-29-5
284 suppliers
$6.00/1g
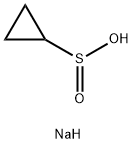
910209-21-1
72 suppliers
$45.00/1g

154350-29-5
284 suppliers
$6.00/1g