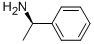
(R)-(+)-1-Phenylethylamine synthesis
- Product Name:(R)-(+)-1-Phenylethylamine
- CAS Number:3886-69-9
- Molecular formula:C8H11N
- Molecular Weight:121.18

67-56-1
751 suppliers
$9.00/25ml
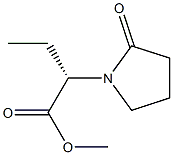
358629-51-3
20 suppliers
inquiry
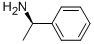
3886-69-9
569 suppliers
$14.00/25G
Yield:358629-51-3 100%
Reaction Conditions:
Stage #1: (-)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacet-N-(+)-(R)-(1-phenylethyl)-amidewith sulfonic acid type-ion exchange resin;water in toluene;not specified; for 6 h;Heating / reflux;
Stage #2: methanolwith sulfonic acid type-ion exchange resin in water;toluene at 60; for 3 h;
Steps:
7
Example 7; (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester.; In a 250 ml reactor equipped with mechanical stirring, thermometer and condenser, 2.5 g of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide (9.112 mmol, d.e.= 99.3%), 24.85 g (6 eq.) of p-toluensulfonic acid supported by polymeric matrix (30.00-60.00 mesh, 2.2 mmol/g) and 75 ml of toluene were charged. To the reaction mixture was added 0.660 ml (36.64 mmol) of water under stirring and mixture was heated up to reflux temperature. Reaction was monitored by HPLC and at complete conversion of starting material (about 6 h), mixture was cooled to 600C temperature and 75 ml of methanol added. Reaction mixture was maintained at that temperature for 3 h up to complete formation of (-)-(S)-alpha- ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester. Reaction mixture was permitted to cool and then it was filtered on gootch in order to separate the product from the resin. Resin was washed with methanol (2 x 75 ml) and organic phases were collected to
References:
WO2008/12268,2008,A1 Location in patent:Page/Page column 20-21
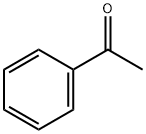
98-86-2
769 suppliers
$9.00/5g
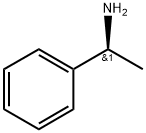
2627-86-3
546 suppliers
$10.60/1gm:
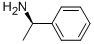
3886-69-9
569 suppliers
$14.00/25G
![2-Propanesulfinamide, 2-methyl-N-(1-phenylethylidene)-, [S(R)]-](/CAS/20200119/GIF/212378-97-7.gif)
212378-97-7
0 suppliers
inquiry
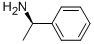
3886-69-9
569 suppliers
$14.00/25G
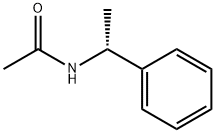
36283-44-0
47 suppliers
$85.00/500mg
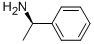
3886-69-9
569 suppliers
$14.00/25G

98-86-2
769 suppliers
$9.00/5g
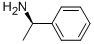
3886-69-9
569 suppliers
$14.00/25G