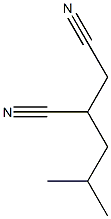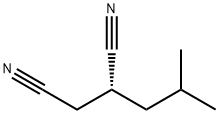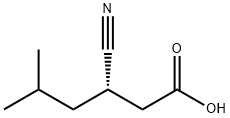
Hexanoic acid, 3-cyano-5-methyl-, (3S)- synthesis
- Product Name:Hexanoic acid, 3-cyano-5-methyl-, (3S)-
- CAS Number:181289-37-2
- Molecular formula:C8H13NO2
- Molecular Weight:155.19
Yield:> 99 % ee
Reaction Conditions:
with nitrilase from Arabis alpina in aq. buffer at 30; pH=8; for 15 h;Resolution of racemate;Green chemistry;Enzymatic reaction;stereoselective reaction;
Steps:
3 Example 3-Application of Nitrilase Aa-Nit-Containing Resting Cells
Kinetic resolution of racemic IBSN. The reaction is shown in FIG. 1. To a Tris-HCl buffer (10 mL, 100 mM, pH 8.0) was added racemic IBSN to reach 1.2 M, and 0.5 g of wet resting cells prepared in Example 1. The mixture was shaken at 30° C. for 15 hr in a water bath shaker. A 500 μL sample was taken every 3 hr and the reaction was quenched with 200 μL of 2 M HCl. The mixture was extracted with 800 μL of ethyl acetate, shaken, and centrifuged (12000×g, 2 min). The supernatant was dried with anhydrous sodium sulfate and analyzed with gas chromatography.
References:
US10100297,2018,B2 Location in patent:Page/Page column 3-5
350801-69-3
0 suppliers
inquiry

181289-37-2
6 suppliers
inquiry
836598-18-6
0 suppliers
inquiry

181289-37-2
6 suppliers
inquiry
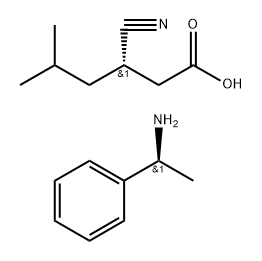
959760-03-3
0 suppliers
inquiry

181289-37-2
6 suppliers
inquiry
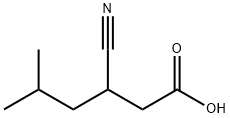
181289-16-7
38 suppliers
inquiry

181289-37-2
6 suppliers
inquiry