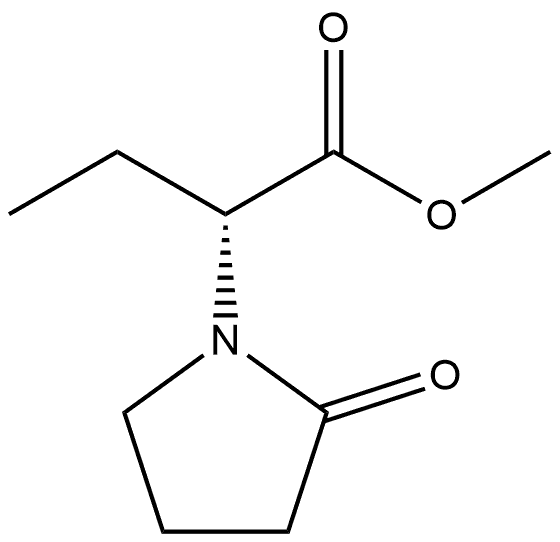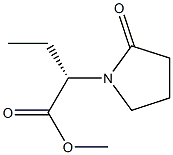
(S)2-(2-Oxo pyrrolidin-1-yl)-Butiric acid methyl ester synthesis
- Product Name:(S)2-(2-Oxo pyrrolidin-1-yl)-Butiric acid methyl ester
- CAS Number:358629-51-3
- Molecular formula:C9H15NO3
- Molecular Weight:185.22

67-56-1
751 suppliers
$7.29/5ml-f

358629-51-3
20 suppliers
inquiry
Yield:358629-51-3 87.5%
Reaction Conditions:
Stage #1:(-)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacet-N-(+)-(R)-(1-phenylethyl)-amide with ethyl-thiophenyl-sulfonic acid supported on silica in toluenesilica; for 5 h;Heating / reflux;
Stage #2:methanol with ethyl-thiophenyl-sulfonic acid supported on silica in toluene at 60; for 3 h;Product distribution / selectivity;
Steps:
9
Example 9; (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetamide (levetiracetam) (alternative 2).; In a 50 ml reactor equipped with mechanical stirring, thermometer and condenser, 0.275 g of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)- amide (1.0 mmol, d.e.= 99.3%), 10.0 g of ethyl-thiophenyl-sulfonic acid supported on silica (0.6 mmol/g, supplied by Phosphonics ) and 15 ml of toluene were charged. To the reaction mixture was added 0.075 ml (4.0 mmol) of water under stirring and mixture was heated up to reflux temperature. Reaction is monitored by HPLC and at complete conversion of starting material (about 5 h), reaction mixture was cooled to 600C temperature and 10 ml of methanol added. Reaction mixture was maintained at that temperature for 3 h up to complete formation of (-)-(S)-alpha-ethyl-2-oxo-l- pyrrolidineacetic acid methyl ester. Reaction mixture was permitted to cool and then worked up according to the procedure described in example 7. 57.9 g of a 0.280% organic solution of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester (0.162 g, 0.875 mmol, 87.5% yield) was thus obtained. Such solution was charged in a flask and concentrated up to a residue was formed. 0.486 g of a brown oil was obtained. Residue was charged in a 5 ml flask equipped with magnetic stirring and condenser. Reaction mixture was cooled to 00C temperature and, keeping under stirring, 1.5 ml of 30% aqueous ammonia solution were charged dropwise. When addition was completed, reaction mixture was thermostabilized at 200C and said conditions were maintained overnight.At complete conversion (about 15 h) excess of ammonia was eliminated by vacuum evaporator. Reaction mixture was then extracted with dichloromethane as described in example 8. Recrystallization of the crude product from refluxing acetone afforded 0.076 g of levetiracetam (0.447 mmol, 44.6% yield compared to the starting amide, e.e. 99.9%).
References:
ZACH SYSTEM S.P.A. WO2008/12268, 2008, A1 Location in patent:Page/Page column 22

67-56-1
751 suppliers
$7.29/5ml-f

358629-51-3
20 suppliers
inquiry
3886-69-9
569 suppliers
$14.00/25G

67-56-1
751 suppliers
$7.29/5ml-f
102849-49-0
162 suppliers
$95.00/100mg

358629-51-3
20 suppliers
inquiry
![Butanoic acid, 2-[(4-ethoxy-4-oxobutyl)amino]-, methyl ester, (2S)-](/CAS/20210305/GIF/497250-64-3.gif)