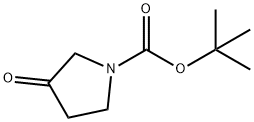
N-Boc-3-pyrrolidinone synthesis
- Product Name:N-Boc-3-pyrrolidinone
- CAS Number:101385-93-7
- Molecular formula:C9H15NO3
- Molecular Weight:185.22
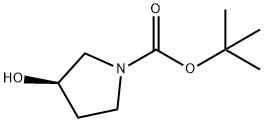
109431-87-0
362 suppliers
$5.00/1g

101385-93-7
476 suppliers
$5.00/1g
Yield:101385-93-7 77.3%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 0 - 20; for 0.2 h;Inert atmosphere;
Steps:
1 Synthesis of tert-butyl 3-oxopyrrolidine-l-carboxylate:
To a stirred solution of tert-butyl (R)-3-hydroxypyrrolidine-l-carboxylate (Intermediate 8-step 1, 4.5 g, 24.1 mmol) in DCM (60 ml) under N2atmosphere, was added DMP (20.45 g, 48.12 mmol) at 0 °C. The reaction mixture was allowed to room temperature and stirred for about 2 hours. After completion of the reaction (monitored by TLC), the reaction mixture was quenched with 1 : 1 mixture of saturated NaHC03and saturated Na2S203solution. The reaction mixture was extracted with DCM (2x100 ml). The combined organic layer was washed with brine, dried over anhydrous sodium sulphate and concentrated under reduced pressure to give the residue. Purification by column chromatography with EtOAc and hexane (15:85) to afford the desired compound (3.44 g, yield: 77.3%) as an oil. 1H NMR (300 MHz, CDC13): δ 3.80-3.75 (m, 4H), 2.61-2.56 (m, 2H), 1.48 (s, 9H)
References:
HETERO RESEARCH FOUNDATION;BANDI, Parthasaradhi Reddy;KURA, Rathnakar Reddy;ADULLA, Panduranga Reddy;GAZULA LEVI, David Krupadanam;MUKKERA, Venkati;NEELA, Sudhakar;LANKA, Vl Subrahmanyam WO2017/17630, 2017, A1 Location in patent:Page/Page column 56
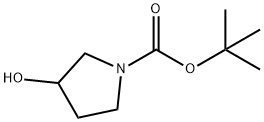
103057-44-9
242 suppliers
$19.00/5g

101385-93-7
476 suppliers
$5.00/1g
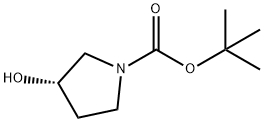
101469-92-5
354 suppliers
$6.00/5g

101385-93-7
476 suppliers
$5.00/1g
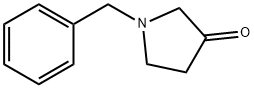
775-16-6
321 suppliers
$31.80/1gm:
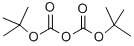
24424-99-5
833 suppliers
$13.50/25G

101385-93-7
476 suppliers
$5.00/1g
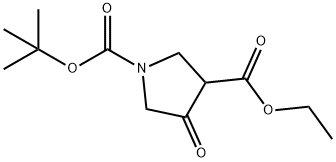
146256-98-6
224 suppliers
$6.00/1g

101385-93-7
476 suppliers
$5.00/1g