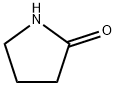
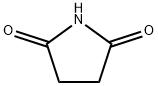
2-Pyrrolidinone synthesis
- Product Name:2-Pyrrolidinone
- CAS Number:616-45-5
- Molecular formula:C4H7NO
- Molecular Weight:85.1

123-75-1
543 suppliers
$10.00/5g

616-45-5
383 suppliers
$14.00/25g
Yield:616-45-5 97%
Reaction Conditions:
with 5.4 wt% Au/CeO2;water monomer;oxygen in diethylene glycol dimethyl ether at 160; under 760.051 Torr; for 6.5 h;Catalytic behavior;Schlenk technique;Reagent/catalyst;
Steps:
2.7 General Procedure for the Au/CeO2-Catalyzed Conversion of Cyclic Secondary Amines to Lactams, in the Presence of O2, as Illustrated with Pyrrolidine
A 100-mL Schlenk flask, equipped with a high-vacuumTeflon stopcock, was charged with a stir bar and 70 mg of5.4 wt% Au/CeO2 catalyst (3.78 mg, 0.0192 mmol of Au).This was followed by the addition of 111 mg of Aerosil 200(amorphous fumed silicon dioxide), 0.45 mL of ultra-purewater, 1.11 mL of a 400 mM stock solution of pyrrolidine(0.444 mmol) in diglyme solvent, and 3.33 mL of a 23.8-mMdodecane stock solution (0.0793 mmol internal standard) indiglyme. The reaction flask was purged through the side armwith oxygen for 1 min and sealed with the stopcock. (A pureoxygen atmosphere, achieved by a more rigorous exclusionof air, led to lower lactam yields and the formation ofa variety of unidentified products, in addition to the lactam. None of the by-products were identified). The contents ofthe sealed flask were stirred at 160 °C in an oil bath. Themole ratio of gold atoms to substrate was 1:23. To monitorthe course of the reaction, the mixture was cooled periodicallyto ambient temperature and an aliquot was withdrawnfor GC analysis. Then the reaction flask was purged againwith O2, re-sealed, and re-heated to 160 °C. After 6.5 h ofreaction time, 100 % substrate conversion was achieved,with a 97 % yield of 2-pyrrolidone. No peroxides (IndigoInstruments peroxide test strips) were detected in the finalreaction mixture. Catalytic conversions of the intermediatesamidine-5 (I) and [4-amino-1-(pyrrolidin-1-yl)butan-1-one] (II) into 2-pyrrolidone followed the same procedure.When the reaction of pyrrolidine was carried out under anair atmosphere, the same procedure was followed as above,but without the oxygen gas purge.
References:
Dairo, Taiwo O.;Nelson, Nicholas C.;Slowing, Igor I.;Angelici, Robert J.;Woo, L. Keith [Catalysis Letters,2016,vol. 146,# 11,p. 2278 - 2291]
96-48-0
3 suppliers
$24.60/25g

616-45-5
383 suppliers
$14.00/25g
541-59-3
367 suppliers
$8.00/5g

616-45-5
383 suppliers
$14.00/25g
2226-88-2
172 suppliers
$345.00/100g

616-45-5
383 suppliers
$14.00/25g
123-56-8
499 suppliers
$6.00/25g

616-45-5
383 suppliers
$14.00/25g