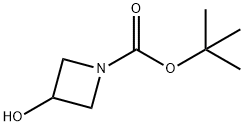
1-N-Boc-3-hydroxyazetidine synthesis
- Product Name:1-N-Boc-3-hydroxyazetidine
- CAS Number:141699-55-0
- Molecular formula:C8H15NO3
- Molecular Weight:173.21

24424-99-5
833 suppliers
$13.50/25G
90604-02-7
222 suppliers
$7.00/10g

141699-55-0
391 suppliers
$6.00/1g
Yield:141699-55-0 357 g
Reaction Conditions:
Stage #1: 1-(diphenylmethyl)-3-azetidinol hydrochloridewith sodium carbonate in dichloromethane;water at 20;
Stage #2: di-tert-butyl dicarbonatewith palladium on carbon;hydrogen in tetrahydrofuran at 20; under 1551.49 Torr; for 18 h;
Steps:
2.2 Step 2. tert-Butyl 3-hydroxyazetidine-l -carboxylate (10)
Step 2. tert-Butyl 3-hydroxyazetidine-l -carboxylate (10) A suspension of l-benzhydrylazetidin-3-ol hydrochloride (9, 625 g, 2.27 mol) in a 10 % solution of aqueous sodium carbonate (Na2C03, 5 L) and dichloromethane (CH2CI2, 5 L) was stirred at room temperature until all solids were dissolved. The two layers were separated, and the aqueous layer was extracted with dichloromethane (CH2CI2, 2 L). The combined organics extracts were dried over sodium sulfate (Na2SC>4) and concentrated under reduced pressure. This resulting crude free base of 9 was then dissolved in THF (6 L) and the solution was placed into a large Parr bomb. Di-teri-butyl dicarbonate (BOC20, 545 g, 2.5 mol, 1.1 equiv) and 20 % palladium (Pd) on carbon (125 g, 50 % wet) were added to the Parr bomb. The vessel was charged to 30 psi with hydrogen gas () and stirred under steady hydrogen atmosphere (vessel was recharged three times to maintain the pressure at 30 psi) at room temperature for 18 h. When HPLC showed that the reaction was complete (when no more hydrogen was taken up), the reaction mixture was filtered through a Celite pad and the Celite pad was washed with THF (4 L). The filtrates were concentrated under reduced pressure to remove the solvent and the residue was loaded onto a Biotage 150 column with a minimum amount of dichloromethane (CH2CI2). The column was eluted with 20 - 50 % ethyl acetate in heptane and the fractions containing the pure desired product (10) were collected and combined. The solvents were removed under reduced pressure to afford terr-butyl 3-hydroxyazetidine-l - carboxylate (10, 357 g, 393.2 g theoretical, 90.8% yield) as colorless oil, which solidified upon standing at room temperature in vacuum. For 10: 'iTNMR (CDCI3, 300 MHz), δ 4.56 (m 1 H), 4.13 (m, 2H), 3.81 (m, 2H), 1.43 (s, 9H) ppm.
References:
WO2013/36611,2013,A1 Location in patent:Page/Page column 27
18621-17-5
378 suppliers
$5.00/1g

24424-99-5
833 suppliers
$13.50/25G

141699-55-0
391 suppliers
$6.00/1g

24424-99-5
833 suppliers
$13.50/25G
18621-18-6
459 suppliers
$8.00/5g

141699-55-0
391 suppliers
$6.00/1g
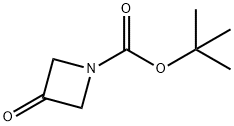
398489-26-4
427 suppliers
$9.00/5g

141699-55-0
391 suppliers
$6.00/1g
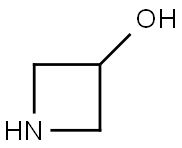
45347-82-8
137 suppliers
$45.00/50mg

24424-99-5
833 suppliers
$13.50/25G

141699-55-0
391 suppliers
$6.00/1g