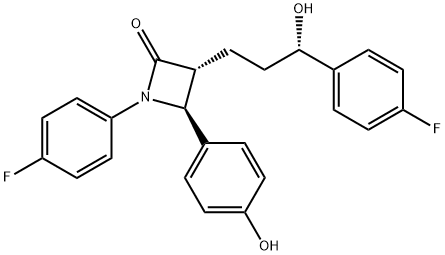
Ezetimibe synthesis
- Product Name:Ezetimibe
- CAS Number:163222-33-1
- Molecular formula:C24H21F2NO3
- Molecular Weight:409.43
163222-32-0
148 suppliers
inquiry

163222-33-1
614 suppliers
$5.00/50mg
Yield:163222-33-1 99%
Reaction Conditions:
with 10 wt% Pd(OH)2 on carbon;hydrogen in methanol;cyclohexane at 70; under 760.051 Torr; for 3 h;
Steps:
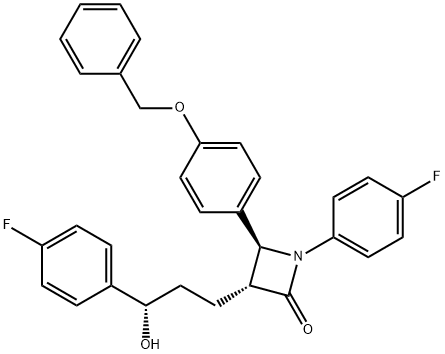
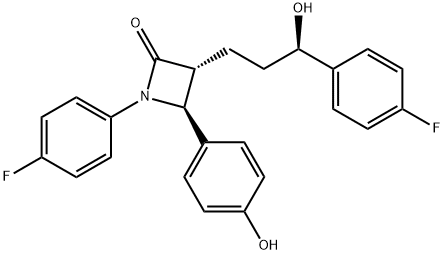
7 Step (7): Synthesis of (3R,4S)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)azetidin-2-one (Compound 11)
(3R,4S)-4-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)azetidin-2-one (10, 258 mg, 0.5 mmol), 20% Pd(OH)2/C (10.53 mg, 15 mol %) and cyclohexane (55.71 μL, 0.55 mmol) were added to a MeOH solvent (6.25 mL) under H2 (1 atm) and then reacted at 70° C. for 3 hr [deprotection]. After completion of the reaction, the reaction product was cooled to room temperature and then filtered through celite using ethyl acetate (25 mL). The filtered solution was concentrated and then recrystallized two times with MeOH and H2O (1/3, 3 mL/9 mL). The obtained solid was filtered using H2O (20 mL), thereby yielding a desired compound (3R,4S)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)azetidin-2-one (11, 203 mg, 99%).(3R,4S)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-hydroxyphenyl)azetidin-2-one (11) 203 mg, 99%, White solid; Rf=0.2 (ethyl acetate:hexane=1:1); 99% ee; [α]20D -28.05 (c 0.15, MeOH) [lit.1 -28.1 (c 0.15, MeOH); 1H NMR (400 MHz, CD3OD) δ 7.34-7.27 (m, 4H), 7.21 (d, J=8.5 Hz, 2H), 7.06-6.96 (m, 4H), 6.81 (d, J=8.5 Hz, 2H), 4.87 (s, 2H), 4.74 (d, J=2.0 Hz, 1H), 4.63-4.60 (m, 1H), 3.10-3.07 (m, 1H), 1.97-1.84 (m, 4H); 13C{1H} NMR (100 MHz, CD3OD) δ 169.9, 163.5 (J=243.6 Hz), 160.5 (J=242.0 Hz), 159.0, 142.2 (J=3.0 Hz), 135.3 (J=2.7 Hz), 129.5, 128.8 (J=8.0 Hz), 128.6, 119.9 (J=7.9 Hz), 117.0, 116.6 (J=22.9 Hz), 115.9 (J=21.6 Hz), 73.8, 62.3, 61.2, 37.5, 26.1; 19F NMR (376 MHz, CD3OD) δ -117.77, -120.15; IR (KBr) 2947, 2924, 1722, 1604, 1509, 1391, 1223 cm-1; HRMS (EI) m/z: [M]+ Calcd for C24H21F2NO3 409.1489; Found 409.1488.
References:
Kangwon National University, University Industry Cooperation Foundation;Lee, Phil Ho;Lee, Koo Yeon;Baek, Yonghyeon;Um, Kyusik;Kim, Byeong Su US2019/256479, 2019, A1 Location in patent:Paragraph 0080-0082
191330-56-0
132 suppliers
$265.00/250mg

163222-33-1
614 suppliers
$5.00/50mg

191330-56-0
132 suppliers
$265.00/250mg

163222-33-1
614 suppliers
$5.00/50mg
163380-16-3
87 suppliers
$145.00/2mg
![Carbonic acid, 4-[(1S,2R,5S)-5-(4-fluorophenyl)-1-[(4-fluorophenyl)amino]-2-[[(4S)-2-oxo-4-phenyl-3-oxazolidinyl]carbonyl]-5-[(trimethylsilyl)oxy]pentyl]phenyl phenylmethyl ester](/CAS/20210305/GIF/1197343-05-7.gif)
1197343-05-7
0 suppliers
inquiry

163222-33-1
614 suppliers
$5.00/50mg
![(3R,4S)-4-[4-(Benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one](/CAS/GIF/190595-65-4.gif)
190595-65-4
187 suppliers
$11.00/250mg

163222-33-1
614 suppliers
$5.00/50mg

163380-16-3
87 suppliers
$145.00/2mg