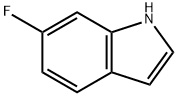
6-Fluoroindole synthesis
- Product Name:6-Fluoroindole
- CAS Number:399-51-9
- Molecular formula:C8H6FN
- Molecular Weight:135.14
![Hydrazinecarboxamide, 2-[2-(4-fluoro-2-nitrophenyl)ethylidene]-, (2E)-](/CAS/20210305/GIF/1065183-63-2.gif)
1065183-63-2
0 suppliers
inquiry

399-51-9
345 suppliers
$11.00/5g
Yield:399-51-9 87%
Reaction Conditions:
with hydrogen;iron(II) acetate;5% rhodium-on-charcoal in tetrahydrofuran;toluene at 20; under 38002.6 Torr; for 48 h;Inert atmosphere;
Steps:
110
Intermediate 110: 6-Fluoro-1H-indole. To a stirred suspension of (1£)-(4-fluoro-2-nitrophenyl)ethanal semicarbazone (27.0 g, 0.1 10 mol) in THF (750 ml.) in a 1 L bomb was added Rh/C (5 %; 3.5 g, 0.002 mol Rh) slurried in toluene and Fe(OAc)2 (2.9 g, 0.017 mol). The bomb was charged with H2 to 50 atm and stirred at room temperature for 2 days. The reaction mixture was filtered through celite, washing with MeOH (150 ml_). The filtrate was concentrated to give a black oil that was partitioned between DCM (500 ml.) and water (300 ml_). The layers were separated and the aqueous fraction was extracted with DCM (2 x 200 ml_). The combined organic extracts were washed with brine (200 ml_), dried (Na2SO4), and concentrated under reduced pressure. The residue was dissolved in DCM (100 ml.) and treated with flash silica to decolourise the solution. The mixture was filtered concentrated under reduced pressure, giving 12.9 g (87 %) of the title compound. 1H NMR: (300 MHz; DMSO-d6) δ 11.1 1 (1 H, s), 7.49 (1 H, dd, J=8.6 & 5.5), 7.30 (1 H, t, J=2.4), 7.13 (1 H, dd, J=10.3 & 2.4), 6.81 (1 H, ddd, J=1 1.0, 7.6 & 2.4) and 6.40-6.38 (1 H, m).
References:
WO2008/118724,2008,A1 Location in patent:Page/Page column 126
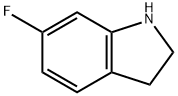
2343-23-9
108 suppliers
$65.00/50mg

399-51-9
345 suppliers
$11.00/5g
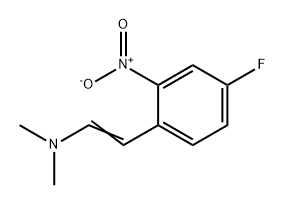
120192-70-3
0 suppliers
inquiry

399-51-9
345 suppliers
$11.00/5g
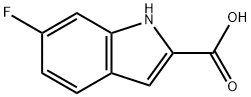
3093-97-8
169 suppliers
$13.00/250mg

399-51-9
345 suppliers
$11.00/5g
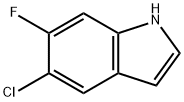
169674-57-1
85 suppliers
$27.00/100mg

399-51-9
345 suppliers
$11.00/5g