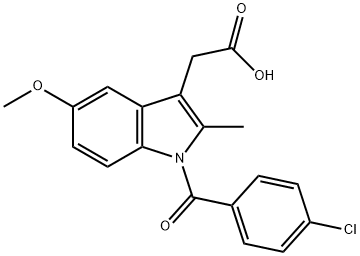
Indometacin synthesis
- Product Name:Indometacin
- CAS Number:53-86-1
- Molecular formula:C19H16ClNO4
- Molecular Weight:357.79

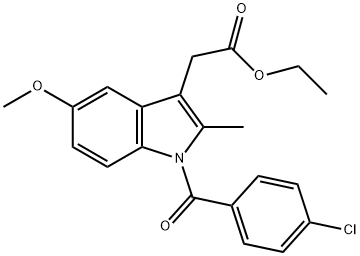
16401-99-3
48 suppliers
inquiry

53-86-1
630 suppliers
$18.17/1g
Yield:53-86-1 657 mg
Reaction Conditions:
with lithium hydroxide monohydrate in water at 25; for 6 h;
Steps:
Indomethacin(10)
LiOH·H2O(0.2 mmol,) in H2O (0.5 mL) was added and continuously stirred for 6h at 25 °C followed by extraction of the organic phase with 2M aq. NH3(2x0.1mL). The aqueous phase was washed withCH2Cl2(10 mL). Addition of conc. HCl (0.2 mL) resulted inprecipitation of the product which was filtered off and washed with water. Theproduct was dissolved in a mixture of MeOH/CH2Cl2 (1 mL),dried over Na2SO4. Filtration and evaporation of thesolvent afforded indomethacin (10;657mg).1H NMR (400 MHz, CDCl3)δ 7.68 - 7.63 (m, 2H), 7.50 - 7.44 (m, 2H), 6.95 (d, J = 2.5, 1H), 6.85(d, J = 9.0, 1H), 6.67 (dd, J = 9.0, 2.5, 1H), 3.83 (s, 3H), 3.70(s, 2H), 2.39 (s, 3H).13C NMR (101 MHz, CDCl3)δ 176.22, 168.44, 156.23, 139.51, 136.42, 133.95, 131.35, 130.93, 130.60,129.30, 115.16, 111.94, 111.86, 101.39, 55.88, 30.05, 13.44.
References:
Gong, Tian-Jun;Cheng, Wan-Min;Su, Wei;Xiao, Bin;Fu, Yao [Tetrahedron Letters,2014,vol. 55,# 11,p. 1859 - 1862] Location in patent:supporting information
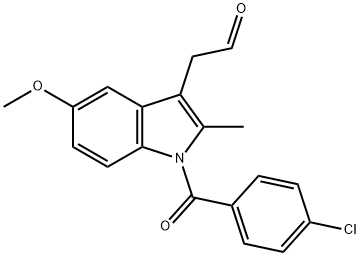
24438-49-1
0 suppliers
inquiry

53-86-1
630 suppliers
$18.17/1g
![Methanone, (4-chlorophenyl)[3-(2-hydroxyethyl)-5-methoxy-2-methyl-1H-indol-1-yl]-](/CAS/20210111/GIF/16130-32-8.gif)
16130-32-8
0 suppliers
inquiry

53-86-1
630 suppliers
$18.17/1g
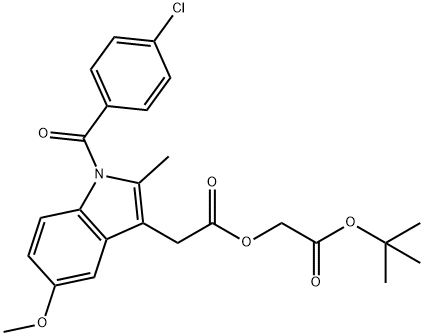
75302-98-6
18 suppliers
inquiry

53-86-1
630 suppliers
$18.17/1g
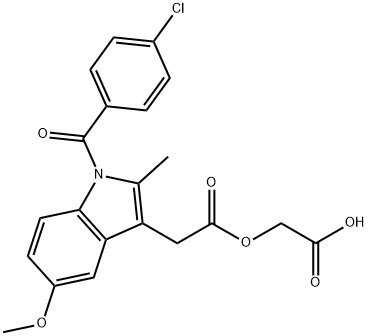
53164-05-9
263 suppliers
$5.00/100mg
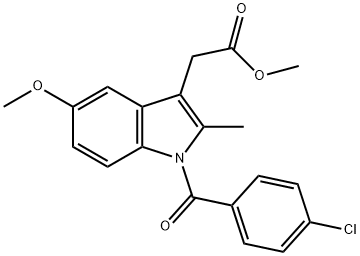
1601-18-9
84 suppliers
$52.00/100mg

53-86-1
630 suppliers
$18.17/1g