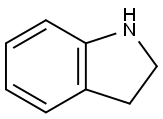
Indoline synthesis
- Product Name:Indoline
- CAS Number:496-15-1
- Molecular formula:C8H9N
- Molecular Weight:119.16
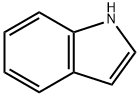
120-72-9
632 suppliers
$6.00/25g

496-15-1
422 suppliers
$8.00/10g
Yield:496-15-1 99%
Reaction Conditions:
with hydrogen in water at 100; under 22502.3 Torr; for 10 h;
Steps:
3.2. Hydrogenation reaction in aqueous phase
General procedure: To showcase the applicability of this novel catalyst, the hydrogenationwas utilized for the other N-heterocyclic compounds. Asshown in Table 4, the hydrogenation of pyridine and 4-methylpyridinecould afford the desired product in excellent yield without by-products(Table 4, entries 1 and 2) although the reactivity of 4-methylpyridinewas somewhat less than that of pyridine, and a slightly longer reactiontime (7.5 h) was needed for full conversion (Table 4, entry 2). Additionally,indole was entirely transformed to dihydroindole and quinolinewas completely converted to 1,2,3,4-tetrahydroquinoline withhigh selectivity, respectively (Table 4, entries 3 and 4).
References:
Qian, Wei;Lin, Lina;Qiao, Yunxiang;Zhao;Xu, Zichen;Gong, Honghui;Li;Chen;Huang, Rong;Hou, Zhenshan [Applied Catalysis A: General,2019,vol. 585,art. no. 117183]
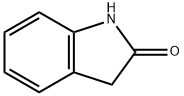
59-48-3
464 suppliers
$5.00/1g

496-15-1
422 suppliers
$8.00/10g
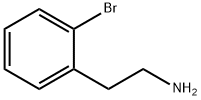
65185-58-2
174 suppliers
$10.00/1g

496-15-1
422 suppliers
$8.00/10g
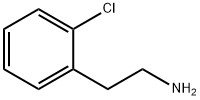
13078-80-3
192 suppliers
$10.00/1g

496-15-1
422 suppliers
$8.00/10g

120-72-9
632 suppliers
$6.00/25g
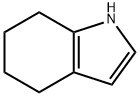
13618-91-2
81 suppliers
$60.00/100mg

496-15-1
422 suppliers
$8.00/10g