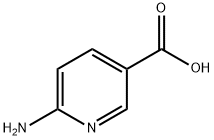
6-Aminonicotinic acid synthesis
- Product Name:6-Aminonicotinic acid
- CAS Number:3167-49-5
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
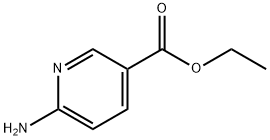
39658-41-8
134 suppliers
$6.00/250mg

3167-49-5
385 suppliers
$8.00/5g
Yield:3167-49-5 33%
Reaction Conditions:
with water;sodium hydroxide in methanol at 20; for 18 h;
Steps:
1.80 Experimental Procedure:
Ethyl 6-aminonicotinate (831.0 mg, 5.00 mmol) was dissolved in methanol (36 mL) and a 1 N solution of NaOH in water (20.0 mL) was added. The reaction mixture was stirred at room temperature for 18 hours. Then, methanol was removed in vacuo and the residue was diluted with water (2 mL). The solution was acidified with 2 N HCL solution in water to a pH of about 3. The precipitate was isolated by centrifugation, washed with water and dried in vacuo. The product was obtained as an off-white solid in 33% yield (229.0 mg) and used in the next step without further purification. 6-Aminonicotinic acid (100.0 mg, 0.72 mmol) was dissolved in DMF (1.5 mL) and triethylamine (201 p L, 1.45 mmol) was added, followed by morpholine (188 μL, 2.18 mmol) and HATU (441.0 mg, 1.16 mmol). The reaction mixture was stirred at room temperature overnight. Ethyl acetate (50 mL) and water (50 mL) were added and the layers were separated. The aqueous layer was extracted with Ethyl Acetate (3×50 mL) and the combined organic layers were washed with water (2×50 mL), brine (1×50 mL), dried over anhydrous sodium sulfate and concentrated in vacuo. The crude product was purified on silica gel with a gradient of DCM and MeOH with 0.1% triethylamine from 100:0 to 20:80. Int-105 was obtained as yellow needles in 66% yield (98.1 mg). 1H-NMR (400 MHz, DMSO-d6): δ 8.84 (bs, 1H), 8.02 (d, J=2.3 Hz, 1H), 7.49-7.45 (m, 1H), 6.50 (bs, 2H), 6.48-6.44 (m, 1H), 3.64-3.55 (m, 4H), 3.53-3.44 (m, 4H) ppm; MS m/z=208.2 [M+H]+.
References:
US2020/299282,2020,A1 Location in patent:Paragraph 0190; 0375
36052-24-1
254 suppliers
$10.00/1g

3167-49-5
385 suppliers
$8.00/5g
1072-98-6
706 suppliers
$5.00/1g
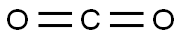
124-38-9
130 suppliers
$214.00/14L

3167-49-5
385 suppliers
$8.00/5g
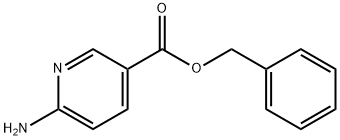
935687-49-3
53 suppliers
$62.00/250mg

3167-49-5
385 suppliers
$8.00/5g
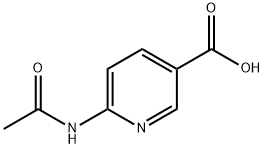
21550-48-1
52 suppliers
$33.00/500mg

3167-49-5
385 suppliers
$8.00/5g