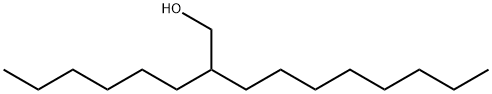
2-Hexyl-1-decanol synthesis
- Product Name:2-Hexyl-1-decanol
- CAS Number:2425-77-6
- Molecular formula:C16H34O
- Molecular Weight:242.44
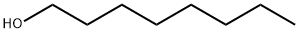
111-87-5
747 suppliers
$6.00/100g

2425-77-6
181 suppliers
$28.89/25ml
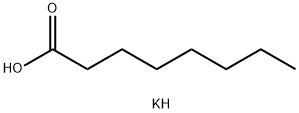
Yield:2425-77-6 74%
Reaction Conditions:
with potassium hydroxide at 190 - 225; for 3 h;Dean-Stark;Inert atmosphere;Reagent/catalyst;Concentration;
Steps:
8 Example 1
General procedure: A 100 mL five-neck flask equipped with; a magnetic stirring-bar, a temperature probe, a nitrogen inlet, a condenser and a Dean-Stark system for separating the water formed by the reaction, was charged with 40 g of 1 -octanol, 0.6 g of granular potassium hydroxide (1 .5 wt%) and 0.4 g of copper-nickel catalyst comprised in a hydrotalcite (the Cu/Ni molar ratio was 2.5/7.5; and the total content of copper and nickel metals in the whole catalyst was 13% by weight). The temperature was elevated under N2 flow (rate of 50 to 60 mL/min) up to the boiling point of the alcohol (195 9C). The time when the reaction mixture reached 190 to 200 QC (reflux) was designed as the point of initiation of the reaction. The highest temperature allowed for the reaction to be conducted was 225 QC. The reaction was terminated after 8 hours. Then, the liquid reaction mixture was cooled and centrifuged to remove the copper- nickel catalyst and the precipitated soap-type products (potassium carboxylates). The composition of the final reaction mixture was analysed by GC, as follow: 83.4% 2-hexyl-1 - decanol; 5.3% C16-non-Guerbet; 5.3% C24-products; 2.1 % 1 -octanol. (0223) The 1 -octanol conversion, 2-hexyl-1 -decanol (C16-Guerbet alcohol) product selectivity, C16- Guerbet (alcohol) product yield after 6 hours reaction and the C16-non-Guerbet (alcohol) (such as 2-hexyl-2-decenal, 2-hexyldecanal and 2-hexyl-2-decen-1 -ol) product yield after 6 hours reaction are shown in Table 1 . A purity of the final target product (2-hexyl-1 -decanol (C16-Guerbet alcohol)) was calculated considering the losses in the mass balance during the reaction (due to the formation of carboxylic salts and 1 -octanol evaporation) and subtracting the un reacted 1 -octanol. This value is a good agreement with the purity of the Cl 6-Guerbet estimated after distillation of the final reaction mixture.
References:
UNIVERSITEIT GENT;VERBERCKMOES, An;VAN DER VOORT, Pascal;HERNáNDEZ, Willinton Yesid;DE VLIEGER, Kevin WO2017/93473, 2017, A1 Location in patent:Page/Page column 17-18; 23
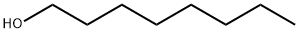
111-87-5
747 suppliers
$6.00/100g

2425-77-6
181 suppliers
$28.89/25ml
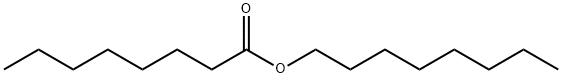
2306-88-9
37 suppliers
$50.00/1 SAMPLE-K

112-30-1
473 suppliers
$5.00/25g

111-27-3
509 suppliers
$5.00/25g
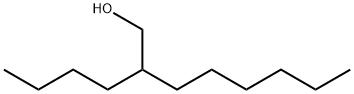
3913-02-8
121 suppliers
$10.00/1g

2425-77-6
181 suppliers
$28.89/25ml
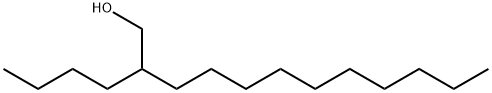
21078-85-3
0 suppliers
inquiry
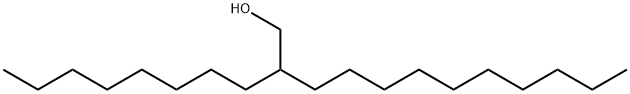
5333-42-6
264 suppliers
$30.00/10g
111-87-5
747 suppliers
$6.00/100g
111-65-9
264 suppliers
$12.00/25g
629-62-9
203 suppliers
$6.00/25g

2425-77-6
181 suppliers
$28.89/25ml