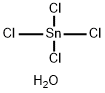
????? 5? C??? ??, ??, ??
??? ??
White solid. Soluble in water or alcohol.
??
Substitute for anhydrous stannic chloride where the presence of water is not objectionable
?? ??
Stannic chloride pentahydrate is a white colored solid. Stannic chloride pentahydrate is soluble in water. Stannic chloride pentahydrate is toxic by ingestion, inhalation and skin absorption. Stannic chloride pentahydrate is used to make perfumes and dyes.
??? ?? ??
Soluble in water. Reacts with water to form Hydrochloric Acid in dense white fumes [Merck 11th ed. 1989].
?? ???
Acidic salts, such as STANNIC CHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
???
Toxic material
????
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
????
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A poison by
intraperitoneal and intravenous routes.
Mutation data reported. A corrosive liquid.
When heated to decomposition it emits
toxic vapors of tin and Cl-.
??? ??
Hydrated stannic chloride is used for fixing certain textile dyes, and for treating silk to give weight to the fabric.
?? ??
UN2440 Stannic chloride, pentahydrate, Hazard class: 8; Labels: 8-Corrosive material.
? ???
Reacts violently with water, forming corrosive hydrochloric acid and tin oxide fumes. Reacts with turpentine, alcohols, and amines, causing fire and explosion hazard. Attacks many metals; some forms of plastics, rubber, and coatings. Reacts with moist air to form hydrochloric acid and dense white fume.
????? 5? ?? ?? ? ???
???
?? ??