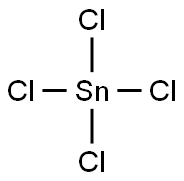
?????
|
|
????? ??
- ???
- -33 °C (lit.)
- ?? ?
- 114 °C (lit.)
- ??
- 2.226 g/mL at 25 °C (lit.)
- ?? ??
- 9 (vs air)
- ???
- 10 mm Hg ( 10 °C)
- ???
- 1.512
- ???
- 34 °F
- ?? ??
- Store at RT.
- ???
- ???, ??, ???, ?????, ???, ??????, ??? ? ?????? ?? ?????.
- ??? ??
- ??
- Specific Gravity
- 2.226
- ??
- ???
- ??????(pH)
- 0.2 (60g/l, H2O, 20℃)Hydrolysis
- ???
- ?? ??
- Hydrolytic Sensitivity
- 8: reacts rapidly with moisture, water, protic solvents
- ??
- Air Sensitive
- Merck
- 14,8774
- ?? ??
- ACGIH: TWA 50 ppm
OSHA: TWA 25 ppm; STEL 125 ppm
NIOSH: IDLH 2300 ppm
- Dielectric constant
- 3.2(22℃)
- ???
- ??? ?????? ?? ??? ?? ???? ??? ? ????. ???, ???? ???? ????.
- LogP
- -1.67 at 20℃
- CAS ??????
- 7646-78-8(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | C,T,F,N | ||
|---|---|---|---|
| ?? ???? ?? | 34-52/53-40-23/24/25-11-67-37-65-50/53 | ||
| ????? | 26-45-61-7/8-36/37-24/25-23-36/37/39-16-62 | ||
| ????(UN No.) | UN 3264 8/PG 2 | ||
| WGK ?? | 2 | ||
| RTECS ?? | XP8750000 | ||
| F ?????? | 10-21 | ||
| TSCA | Yes | ||
| ?? ?? | 8 | ||
| ???? | II | ||
| HS ?? | 28273990 | ||
| ?? ?? ??? | 7646-78-8(Hazardous Substances Data) | ||
| ???? ?? | KE-33876 | ||
| ?????? ??? | 97-1-93 | ||
| ?? ? ???? | ????: ????; ???(??)????: ???? ?? ? ?? 25% ?? ??? ??? |
????? C??? ??, ??, ??
??
Tin (IV) chloride appears as white crystals with a strong pungent chlorine odour. On heating, tin (IV) chloride decomposition emits acrid fumes. At room temperature, it is colourless and releases fumes on contact with air, giving a stinging odour. Stannic chloride was used as a chemical weapon during World War I. It is also used in the glass container industry for making an external coating that toughens the glass. Stannic chloride is used in chemical reactions with fuming (90%) nitric acid for the selective nitration of activated aromatic rings in the presence of unactivated ones. Tin (IV) chloride reacts violently with water or moist air to produce corrosive hydrogen chloride. Tin (IV) chloride reacts with turpentine, alcohols, and amines, causing fire and explosion hazard. It attacks many metals, some forms of plastic, rubber, and coatings.??? ??
Tin tetrachloride is a colorless fuming liquid.??? ??
Colorless fuming liquid; corrosive; density 2.234 g/mL; freezes at -33°C; boils at 114.15°C; critical temperature 318.75°C; critical pressure 37.98 atm; critical volume 351 cm3/mol; soluble in cold water, evolving heat; decomposed by hot water; soluble in alcohol, benzene, toluene, chloroform, acetone and keroseneThe pentahydrate is a yellowish-white crystalline solid or small, fused lumps; faint odor of HCl; density 2.04 g/cm3; decmposes at 56°C; very soluble in water; soluble in ethanol.
??
Electroconductive and electroluminescent coatings, mordant in dyeing textiles, perfume stabilization, manufacture of fuchsin, color lakes, ceramic coatings, bleaching agent for sugar, stabilizer for certain resins, manufacture of blueprint and other sensitized papers, other tin salts, bacteria and fungi control in soaps?? ??
Tin(II) chloride is prepared by dissolving tin in hydrochloric acid followed by evaporation of the solution and crystallization.??
Often sold in the form of the double salt with sodium chloride: Na2SnCl6?H2O.?? ??
Stannic chloride (SnCl4) is a strong Lewis acid widely used as a promoter or catalyst in organic synthesis. It is soluble in most organic solvents.??? ?? ??
Fumes in moist air. Reacts with water to form Hydrochloric Acid in dense white fumes [Merck 11th ed. 1989].?? ???
Acidic salts, such as STANNIC CHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions (ethylene oxide polymerization). Combination of the chloride with turpentine is strongly exothermic, and may lead to ignition, [Mellor, 1941, Vol. 7, 446].???
Evolves heat on contact with moisture. Corrosive liquid????
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.????
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.Safety Profile
Poison by intraperitoneal route. Moderately toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. Combustible by chemical reaction. Upon contact with moisture, considerable heat is generated. Violent reaction with K, Na, turpentine, ethylene oxide, alkyl nitrates. Dangerous; hydrochloric acid is liberated on contact with moisture or heat. When heated to decomposition it emits toxic fumes of Cl-. See also HYDROCHLORIC ACID.??? ??
Tin tetrachloride is used in the production of blueprints and electroconductive readings, as a bleaching agent for sugar and resin stabilizer.?? ??
UN2440 Stannic chloride, pentahydrate, Hazard class: 8; Labels: 8-Corrosive material.Purification Methods
SnCl4 fumes in moist air due to formation of a hydrate. Fractionate it in a ground glass still and store it in the absence of air. Possible impurities are SO2 and HCl [Baudler in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 729 1963]. It forms a solid pentahydrate [10026-06-9] which smells of HCl and is obtained when the anhydrous salt is dissolved in a small volume of H2O. Also reflux it with clean mercury or P2O5 for several hours, then distil it under (reduced) N2 pressure into a receiver containing P2O5. Finally redistil it. Alternatively, distil it from Sn metal under vacuum in an all-glass system and seal off in large ampoules. SnCl4 is available commercially as 1M solutions in CH2Cl2 or hexane. HARMFUL VAPOURS.? ???
Slowly forms hydrochloric acid in cold water; fast reaction in hot water and steam. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, water, turpentine, potassium, sodium, ethylene oxide; nitrates, alcohols, amines, chlorine, strong acids; strong bases. Attacks metals, rubbers and some plastics in the resence of moisture.??? ??
SnCl4: Pour onto sodium bicarbonate; spray with ammonium hydroxide while adding crushed ice; when reaction subsides, flush down drain.????? ?? ?? ? ???
???
?? ??
1-(5-BROMO-1-BENZOTHIEN-3-YL)???
3-(?????)-2,4,5-???????
Tetrabromophenol Blue
1-??[b]???-3-?-2-?????-1-?
5-???-??[B]???-3-????
(5-???-3-??[B]???)???
1-(5-CHLOROBENZO[B]THIOPHEN-3-YL)ETHANONE
3-(Benzofuran-2-yl)-3-oxopropanenitrile ,97%
2-(N-HEPTANOYL)THIOPHENE
2-(3-?????)????
PLUMBAGIN
??? ??? ?????? ???
1-(1-BENZOFURAN-2-YL)-2-BROMOETHAN-1-ONE
3-????????
2-ETHYL-3-HYDROXY-6-METHYLPYRIDINE
(5-???-1-?????-3-YL)???
5-BROMOBENZO[B]THIOPHENE-3-CARBOXYLIC ACID
?? ????
?????? ????
Azocyclotin suspensoid
3-METHYL-1H-PYRAZOLO[3,4-C]PYRIDAZINE-4,5-DIOL
6-???-4,4-?????-???
2-(8-BROMO-2,3,6,7-TETRAHYDRO-BENZO[1,2-B:4,5-B']DIFURAN-4-YL)-1-METHYL-ETHYLAMINE
2,5-???-3-????
1-Tetralone
5-(2-???)????
4-NITROPHENYL-ALPHA-D-GALACTOPYRANOSIDE
3-????????
2-???????
Azocyclotin W.P.
3-?????[b]???
4,5,6,7-TETRAHYDRO-2-METHYLFURO[2,3-C]PYRIDINE
????? ?? ??
???( 342)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 |
info@dycnchem.com | China | 52846 | 58 |
| Changzhou Zechong Biopharma Co., Ltd. | +undefined18621330623 |
sales@zechongchem.com | China | 28 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085; +8615531157085 |
abby@chuanghaibio.com | China | 8804 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86-13288715578 +86-13288715578 |
sales@hbmojin.com | China | 12765 | 58 |
| Capot Chemical Co.,Ltd. | +8613336195806 |
sales@capot.com | China | 29731 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21623 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
FandaChem@Gmail.com | China | 2273 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2986 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | 86-22-66880623 +8618622897568 |
sales@tjzxchem.com | China | 572 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29856 | 58 |