Dibismuttrioxid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Chemische Eigenschaften
Heavy, Yellow Powder
Physikalische Eigenschaften
Yellow monoclinic crystal or powder; density 8.90 g/cm
3; melts at 817°C; vaporizes at 1,890°C; insoluble in water; soluble in acids.
Occurrence
Bismuth trioxide occurs in nature as mineral bismite. The oxide is used in fireproofing of papers and polymers; in enameling cast iron ceramic; and in disinfectants.
Verwenden
Bismuth(III) oxide is used in the preparation of BiFeO3perovskite nanoparticles. It finds use in disinfectants, magnets, glass, rubber, vulcanization, fireproofing papers and polymers and catalysts. Bismuth trioxide brings about the "dragon's eggs" effect in fireworks, as a replacement of red lead. Bismuth(III) compounds are attractive reagents and catalysts in organic synthesis because of their low cost and ease of handling. Bismuth trioxide nanoparticles also play an important role in high energy gas generators. The alpha crystalline form of bismuth(III) oxide has p-type electronic conductivity.
synthetische
Bismuth trioxide is commercially made from bismuth subnitrate. The latter is produced by dissolving bismuth in hot nitric acid. Addition of excess sodium hydroxide followed by continuous heating of the mixture precipitates bismuth trioxide as a heavy yellow powder. Also, the trioxide can be prepared by ignition of bismuth hydroxide.
Allgemeine Beschreibung
Bismuth (III) oxide is a yellow, monoclinic crystalline powder. It is insoluble in water and hydroxide solutions but dissolves in acids to form bismuth (III) salts. It can be prepared by heating bismuth in air or by heating hydroxides, carbonates or nitrates of bismuth.
Structure and conformation
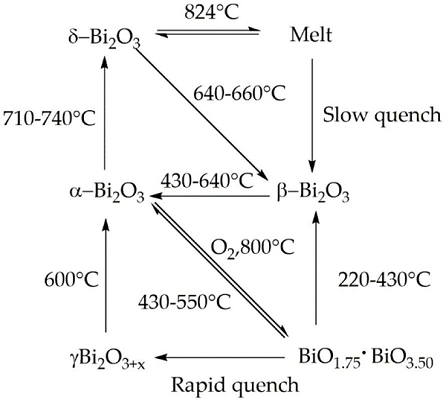
Bismuth(III) Oxides are the deeply studied class of bismuth compounds, and they present four different phases. At room temperature, monoclinic α-Bi2O3 is the common stable phase with a polymeric-distorted layered structure composed of pentacoordinate bismuth atoms enclosed into pseudo-octahedral units. At a temperature higher than 710 °C, the α phase is converted into the cubic δ phase, which has a defective structure with random oxygen vacancies. The β phase and several oxygen-rich forms are closely related to the δ phase. In particular, the vacancy structures of highly defected bismuth oxides are some sites filled with O?2 and Bi(III) and Bi (V) sites. Bismuth oxide γ-phase also shows a cubic structure, but it is highly unstable and hard to synthesize without supporting it onto other oxides or metallic species. The other two polymorphic metastable bismuth oxide phases are known as the ω phase, stable at temperatures higher than 800 °C and the ε phase, isolated in 2006 by Cornei and co-workers[1].
Dibismuttrioxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

