| Identification | More | [Name]
2-Cyclohexen-1-one | [CAS]
930-68-7 | [Synonyms]
2-CYCLOHEXANE-1-ONE
2-CYCLOHEXEN-1-ONE
2-CYCLOHEXENONE
2-CYCLOHEXENONE-1
CYCLOHEX-2-ENONE
Cyclohexenone
TIMTEC-BB SBB008209
1-Cyclohexen-3-one
2-Cyclohexene-1-one
3-oxocyclohexene
Cyclohex-2-en-1-one
Cyclohexen-2-one
2-CYCLOHEXEN-1-ONE, BASF QUALITY
2-CYCLOHEXEN-1-ONE, 95+%
2-Cyclohexen-1-one, 97+%
1-cyclohexen-3-one,2-cyclohexen-1-one,2-cyclohexenone
2-cyclohex
1-CYCLOHEXEN-2-ONE
Cyclohexen-3-one | [EINECS(EC#)]
213-223-5 | [Molecular Formula]
C6H8O | [MDL Number]
MFCD00001577 | [Molecular Weight]
96.13 | [MOL File]
930-68-7.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR LIGHT YELLOW TO YELLOW LIQUID | [Melting point ]
-53 °C | [Boiling point ]
171-173 °C(lit.)
| [density ]
0.993 g/mL at 25 °C(lit.)
| [vapor pressure ]
760 mm Hg ( 168 °C)
| [FEMA ]
4517 | 2-CYCLOHEXENONE | [refractive index ]
n20/D 1.488(lit.)
| [Fp ]
133 °F
| [storage temp. ]
0-6°C | [solubility ]
soluble in Chloroform, Methanol | [form ]
Liquid | [color ]
Clear light yellow to yellow | [Odor]
No strong odour known | [Odor Type]
roasted | [Water Solubility ]
SOLUBLE | [JECFA Number]
2052 | [BRN ]
1280477 | [Contact allergens]
This strong sensitizer has been responsible for chemical burning followed by sensitization in a chemistry student. | [LogP]
0.61 | [CAS DataBase Reference]
930-68-7(CAS DataBase Reference) | [NIST Chemistry Reference]
2-Cyclohexen-1-one(930-68-7) | [EPA Substance Registry System]
930-68-7(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR LIGHT YELLOW TO YELLOW LIQUID | [Definition]
ChEBI: A cyclohexenone having its C2C double bond at the 2-position. | [Description]
2-Cyclohexen-1-one is a ketone, or more precisely an enone. It is colorless liquid, but commercial samples are often yellow. Industrially, cyclohexenone is prepared from phenol by Birch reduction. Common reactions include nucleophilic conjugate addition with organocopper reagents, Michael reactions and Robinson annulations.
| [Uses]
2-Cyclohexen-1-one is used as intermediates. | [Application]
2-Cyclohexen-1-one can be used to versatile electrophile employed in a range of addition reactions including conjugate addition of organocopper nucleophiles, Michael reaction with enol silanes, and phosphoniosilylations. | [Reactions]
2-Cyclohexen-1-one was employed in a Baylis–Hillman-type reaction with formaldehyde, and the newly formed primary alcohol was protected as silyl ether.
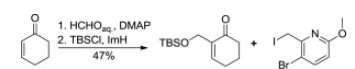
| [Synthesis Reference(s)]
Chemical and Pharmaceutical Bulletin, 31, p. 4209, 1983 DOI: 10.1248/cpb.31.4209
Journal of the American Chemical Society, 110, p. 6591, 1988 DOI: 10.1021/ja00227a065 | [Solubility in organics]
Acetone, Alcohol, Benzene |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R22:Harmful if swallowed.
R23/24:Toxic by inhalation and in contact with skin . | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2929 6.1/PG 2
| [WGK Germany ]
3
| [RTECS ]
GW7000000
| [Hazard Note ]
Toxic | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
29142990 | [Safety Profile]
A poison by ingestion,
inhalation, intraperitoneal, and skin contact
routes. Mutation data reported. When
heated to decomposition it emits acrid
smoke and irritant fumes. See also
KETONES. |
|
|