| Identification | More | [Name]
Cyclohexene | [CAS]
110-83-8 | [Synonyms]
1,2,3,4-TETRAHYDROBENZENE
BENZENETETRAHYDRIDE
CYCLOHEXENE
HX
TETRAHYDROBENZENE
1-Cyclohexene
3,4,5,6-tetrahydrobenzene
Benzene, tetrahydro-
benzene,tetrahydro-
Cyclohex-1-ene
cyclohexenering
Cykloheksen
cykloheksen(polish)
Hexanaphthylene
tetrahydro-benzen
CYCLOHEXENE, REAGENTPLUS, 99%
CYCLOHEXENE, REAGENTPLUS, >=99.0%
CYCLOHEXENE, STAB.
Cyclohexene, pure, 99%
Cyclohexen | [EINECS(EC#)]
203-807-8 | [Molecular Formula]
C6H10 | [MDL Number]
MFCD00001539 | [Molecular Weight]
82.14 | [MOL File]
110-83-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Cyclohexene is a colorless liquid (cyclic
alkene) with a sweetish odor. | [Melting point ]
-104 °C (lit.) | [Boiling point ]
83 °C (lit.) | [density ]
0.811 g/mL at 25 °C(lit.)
| [vapor density ]
2.8 (vs air)
| [vapor pressure ]
160 mm Hg ( 20 °C)
| [refractive index ]
n20/D 1.446(lit.)
| [Fp ]
10 °F
| [storage temp. ]
Flammables area | [solubility ]
water: insoluble | [form ]
Liquid | [color ]
Clear colorless | [Specific Gravity]
0.811 | [PH]
7-8 (0.2g/l, H2O, 20℃) | [Stability:]
Stable in the absence of air-may form peroxides in storage. Incompatible with oxidizing agents. Highly flammable. | [explosive limit]
1.2-7.7%(V) | [Water Solubility ]
insoluble | [Merck ]
14,2727 | [BRN ]
906737 | [Henry's Law Constant]
3.85 x 10-2 atm?m3/mol at 25 °C (Nielsen et al., 1994) | [Dielectric constant]
18.3(20℃) | [Exposure limits]
TLV-TWA 300 ppm (~1015 mg/m3)
(ACGIH, OSHA, and NIOSH); IDLH
10,000 ppm (NIOSH). | [InChIKey]
HGCIXCUEYOPUTN-UHFFFAOYSA-N | [LogP]
2.99 at 25℃ | [CAS DataBase Reference]
110-83-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Cyclohexene(110-83-8) | [EPA Substance Registry System]
110-83-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xn | [Risk Statements ]
R11:Highly Flammable.
R21/22:Harmful in contact with skin and if swallowed .
R65:Harmful: May cause lung damage if swallowed. | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S29:Do not empty into drains .
S33:Take precautionary measures against static discharges .
S36/37:Wear suitable protective clothing and gloves . | [OEB]
A | [OEL]
TWA: 300 ppm (1015 mg/m3) | [RIDADR ]
UN 2256 3/PG 2
| [WGK Germany ]
1
| [RTECS ]
GW2500000
| [Autoignition Temperature]
590 °F | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29021990 | [Safety Profile]
Moderately toxic by
inhalation and ingestion. A very dangerous
fire hazard when exposed to flame; can react
with oxidizers. Dangerous; keep away fromheat and open flame. To fight fire, use foam,
CO2, dry chemical. | [Hazardous Substances Data]
110-83-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 1300 mg/kg | [IDLA]
2,000 ppm |
| Hazard Information | Back Directory | [General Description]
A colorless liquid. Insoluble in water and less dense than water. Flash point 20°F. Vapors heavier than air. Inhalation of high concentrations may have a narcotic effect. Used to make other chemicals. | [Reactivity Profile]
CYCLOHEXENE(110-83-8) may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154]. | [Air & Water Reactions]
Highly flammable. Insoluble in water. | [Health Hazard]
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. | [Potential Exposure]
May be used as an intermediate in
making other chemicals (e.g., adipic acid, maleic acid, hex-
ahydro benzoic acid), oil extraction and as a catalyst
solvent. | [Fire Hazard]
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ-
ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi-
cal attention. Do NOT induce vomiting. | [Shipping]
UN2256 Cyclohexene, Hazard Class: 3; Labels:
3-Flammable liquid. | [Incompatibilities]
Vapor may form explosive mixture with
air. The substance can form explosive peroxides. The sub-
stance may polymerize under certain conditions.
Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong bases, strong acids, oxoa-
cids, epoxides. | [Description]
Cyclohexene is a hydrocarbon, mostly obtained from the
hydrogenation of benzene. | [Waste Disposal]
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinera-
tor equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. | [Physical properties]
Clear, colorless liquid with a sweet odor. Odor threshold concentration is 0.18 ppm (quoted,
Amoore and Hautala, 1983). | [Definition]
ChEBI: A cycloalkene that is cylohexane with a single double bond. | [Production Methods]
Cyclohexene is prepared by dehydration of cyclohexanol by
thermal reaction of an ethylene–propylene–butadiene mixture
(1). | [Carcinogenicity]
Cyclohexene was not mutagenic in Salmonella
typhimurium with or without metabolic
activation. | [Environmental Fate]
Biological. Cyclohexene biodegrades to cyclohexanone (Dugan, 1972; Verschueren, 1983).
Photolytic. The following rate constants were reported for the reaction of cyclohexene with OH
radicals in the atmosphere: 6.80 x 10-11 cm3/molecule?sec (Atkinson et al., 1979), 6.75 x 10-11
cm3/molecule?sec at 298 K (Sablji? and Güsten, 1990), 5.40 x 10-11 cm3/molecule?sec at 298 K
(Rogers, 1989), 1.0 x 10-10 cm3/molecule?sec at 298 K (Atkinson, 1990); with ozone in the gasphase:
1.69 x 10-16 cm3/molecule?sec at 298 K (Japar et al., 1974), 2.0 x 10-16 at 294 K (Adeniji et
al., 1981), 1.04 x 10-16 cm3/molecule?sec (Atkinson et al., 1983), 1.04 x 10-16 at 298 K (Atkinson
and Carter, 1984); with NO3 in the atmosphere: 5.26 x 10-13 cm3/molecule?sec (Sablji? and Güsten,
1990); 5.3 x 10-13 cm3/molecule?sec at 298 K (Atkinson, 1990), and 5.28 x 10-13 cm3/molecule?sec
at 295 K (Atkinson, 1991). Cox et al. (1980) reported a rate constant of 6.2 x 10-11
cm3/molecule?sec for the reaction of gaseous cyclohexene with OH radicals based on a value of 8
x 10-12 cm3/molecule?sec for the reaction of ethylene with OH radicals.
Chemical/Physical. Gaseous products formed from the reaction of cyclohexene with ozone were
(% yield): formic acid , carbon monoxide , carbon dioxide, ethylene, and
valeraldehyde (Hatakeyama et al., 1987). In a smog chamber experiment conducted in the
dark at 25 °C, cyclohexane reacted with ozone. The following products and their respective molar
yields were: oxalic acid (6.16%), malonic acid (6.88%), succinic acid (0.63%), glutaric acid
(5.89%), adipic acid (2.20%), 4-hydroxybutanal (2.60%), hydroxypentanoic acid (1.02%),
hydroxyglutaric acid (2.33%), hydroxyadipic acid (1.19%), 4-oxobutanoic acid (6.90%), 4-
oxopentanoic acid (4.52%), 6-oxohexanoic acid (4.16%), 1,4-butandial (0.53%), 1,5-pentanedial
(0.44%), 1,6-hexanedial (1.64%), and pentanal (17.05%).
Grosjean et al. (1996) investigated the atmospheric chemistry of cyclohexene with ozone and an
ozone-nitrogen oxide mixture under ambient conditions. The reaction of cyclohexene and ozone in
the dark yielded pentanal and cyclohexanone. The sunlight irradiation of cyclohexene with ozonenitrogen
oxide yielded the following carbonyls: formaldehyde, acetaldehyde, acetone, propanal,
butanal, pentanal, and a C4 carbonyl.
Cyclohexene reacts with chlorine dioxide in water forming 2-cyclohexen-1-one (Rav-Acha et
al., 1987). | [storage]
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.May form peroxides in storage. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Cumene must be stored to avoidcontact with oxidizers, such as permanganates, nitrites, peroxides, chlorates, and perchlorates, since violent reactionsoccur. Store in tightly closed containers in a cool well-ventilated area away from heat. Sources of ignition, such assmoking and open flames, are prohibited where Cumene isused, handled, or stored in a manner that could create apotential fire or explosion hazard. Metal containers involving the transfer of=gallons or more of this chemical shouldbe grounded and bonded. Drums must be equipped withself-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of thischemical.chemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containersin a cool, well-ventilated area away from strong oxidizers(such as chlorine, bromine, and fluorine). Sources of ignition, such as smoking and open flames, are prohibitedwhere cyclohexene is handled, used, or stored. Metal containers involving the transfer of=gallons or more of cyclohexene should be grounded and bonded. Drums must beequipped with self-closing valves, pressure vacuum bungs,and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers ofcyclohexene. Wherever cyclohexene is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings. | [Purification Methods]
Free cyclohexene from peroxides by washing with successive portions of dilute acidified ferrous sulfate, or with NaHSO3 solution, then with distilled water, drying with CaCl2 or CaSO4, and distilling under N2. Alternative methods for removing peroxides include passage through a column of alumina, refluxing with sodium wire or cupric stearate (then distilling from sodium). The diene is removed by refluxing with maleic anhydride before distilling under vacuum. Treatment with 0.1moles of MeMgI in 40mL of diethyl ether removes traces of oxygenated impurities. Other purification procedures include washing with aqueous NaOH, drying and distilling under N2 through a spinning band column, redistilling from CaH2, storing under sodium wire, and passing through a column of alumina, under N2, immediately before use. Store it at <0o under argon. [Coleman & Johnstone Org Synth Coll Vol I 83 1955, Carson & Ipatieff Org Synth Coll Vol II 152 1943, Woon et al. J Am Chem Soc 108 7990 1986, Wong et al. J Am Chem Soc 109 3428 1987.] [Beilstein 5 IV 218.] | [Toxicity evaluation]
The release of cyclohexene into the air occurs in the form of
waste streams from manufacturing units. Cyclohexene has
a vapor pressure of 89mm Hg at 25°C, indicating that it exists
as a vapor form in the environment and is degraded by reactions
with photochemically induced hydroxyl radicals, ozone,
and nitrate radicals. The half-life for these reactions in the air is
6, 2, and 4 h, respectively.
Estimated Koc of 850 indicates that cyclohexene has low
mobility in the soil. Cyclohexene has Henry’s law constant of
4.55×10�-2atm-m3 mol-1. Based on this Henry’s law constant,
volatilization is expected to be the major process of removal of
cyclohexene from moist soil and water, if released into it. | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL} for Cyclohexene is 10,000 μg/m3 based on
an 8 hour averaging time. |
| Questions And Answer | Back Directory | [Chemical Properties]
Cyclohexene (C6H10, CAS registry No. 110-83-8) is also called tetrahydrobenzene, which is colorless flammable liquid. It is insoluble in water. Inhalation of high concentrations may have a narcotic effect. Cyclohexene is highly flammable, so it should keep away from heat and open flame. It may form peroxides in storage, so it should be stored in the absence of air. It may cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. | [Cycloolefin]
Cyclohexene is a cyclic olefin, and is a colorless, flammable liquid with a special pungent odor at room temperature. Long-term placement in the air it can be oxidated into peroxide by air. Naturally present in coal tar, soluble in acetone, carbon tetrachloride, benzene, ether, hexane, ethanol and other organic solvents, can form binary azeotrope with lower alcohols, acetic acid, etc. Cyclohexene has the general nature of olefin, decomposes rapidly in the presence of uranium salts under sunlight or ultraviolet rays, is constant as being heated in a sealed tube at 200 ℃ for a long time, forms benzene and naphthalene at 400~500 ℃.
It is obtained by dehydration of cyclohexanol at high temperature in the presence of an acid catalyst in industrial. It is obtained by sulfated dehydration of cyclohexanol in the laboratory.
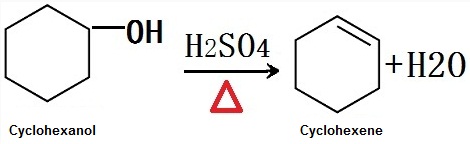
Figure 1 is the chemical reaction equation of sulfated dehydration of cyclohexanol to obtain cyclohexene.
Cyclohexene is an important chemical raw materials, used for the production of adipic acid, adipic aldehyde, maleic acid, Cyclohexane acid, cyclohexane aldehyde, maleic acid, cyclohexyl carboxylic acid, cyclohexanecarboaldehyde in industry. It is also used as the extraction agent, the stabilizer having a high-octane gasoline. Inhalation can cause mild poisoning.
Here are some of these important compounds prepared by cyclohexene, (1)chlorinated cyclohexane is made, useful as pharmaceutical intermediates, a solvent and rubber additives. (2) cyclohexanone is made from cyclohexene, can be used as intermediates raw materials of medicine, pesticides, perfumes, and dyes, as polymer modifiers. (3) cyclohexyl acetate is made, used as a plastic solvent. (4) cyclohexanone phenol is made, can be used as raw materials medicine and pesticides. (5) aminocyclohexanol is made, can be used as surfactants and emulsifiers. (6) The product can also be used directly as organic intermediates, solvents and additives when spices are prepared. It can be used in the preparation of butadiene in the laboratory.
The above information were edited and collated by Yan Yanyong of Chemicalbook.
| [Preparation]
Cyclohexene can be synthesized by several ways, such as dehydrogenation of cyclohexane, dehydration of cyclohexanol, dehydrohalogenation of halogenated cyclohexane, Birch reduction or partial hydrogenation of benzene. Compared with other methods,the technology of partial hydrogenation of benzene to cyclohexene is of the advantage of safety, high atom-economy, environmentally friendly. The partial hydrogenation of benzene to cyclohexene has been known for more than 100 years. | [Chemical properties]
Colorless flammable liquid. Insoluble in water, soluble in ether.
| [Selective Oxidation]
Cyclohexene may react vigorously with strong oxidizing agents. Though simple in its chemical structure, there are two potential oxidation sites in cyclohexene, and the usual oxidation reactions generally lead to a mixture of products with different oxidation states and functional groups: oxidation of the C=C bond (site a) can lead to 7-oxabicyclo[4.1.0]heptane, trans/cis-cyclohexane-1,2-diol, or adipic acid; oxidation at the allylic C-H position (site b) may produce cyclohex-2-en-1-ol or cyclohex-2-en-1-one. These products are useful industrial intermediates that have been widely employed in organic synthesis, medicinal chemistry, pesticide chemistry, materials science, etc. Therefore, controllable oxidation reactions for cyclohexene that can selectively afford the targeted products are synthetically valuable for applications in both the academy and industry, thus becoming the aim of synthetic and catalytic chemists in the field. | [Uses]
- Used in organic synthesis, it is also used as a solvent.
- Cyclohexene is used as organic synthetic raw materials, such as synthetic raw materials for lysine, cyclohexanone, phenol, polycycloolefin resin, chlorinated cyclohexane, rubber additives, cyclohexanol, etc. It is also used as a catalyst solvent and petroleum extraction agent, high octane gasoline stabilizer.
- Cyclohexene is used for Preparation of adipic acid, maleic acid, hexahydro benzoic acid and acetaldehyde, preparation of butadiene in the laboratory. It is used as high-octane gasoline stabilizer.
| [Production method]
Cyclohexanol is heated to generate cyclohexene in the presence of sulfuric acid catalyst, distilled to obtain crude products. Washed with a fine saturated salt solution, then sodium sulfate solution is used to neutralize traces of acid, then washed with water, layered, dried, filtration, distillation, collecting 82-85 ℃ distillate products to obtain cyclohexene.
| [Flammability hazard characteristics]
In case of fire, high temperature, oxidant, it is flammable, burning produces irritant smoke.
| [Storage Characteristics]
Treasury ventilation low-temperature drying, stored separately from oxidants and acids. Not long storage to prevent polymerization.
|
|
|