| Identification | More | [Name]
Testosterone | [CAS]
58-22-0 | [Synonyms]
17BETA-HYDROXY-3-OXO-4-ANDROSTENE
17BETA-HYDROXY-4-ANDROSTEN-3-ONE
17B-HYDROXY-4-ANDROSTEN-3-ONE
17b-hydroxyandrost-4-ene-3-one
4-ANDROSTEN-17BETA-OL-3-ONE
4-ANDROSTENE-17BETA-OL-3-ONE
(8R,9S,10R,13S,14S,17S)-17-HYDROXY-10,13-DIMETHYL-1,2,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-CYCLOPENTA[A]PHENANTHREN-3-ONE
android
androlin
androst-4-en-17b-ol-3-one
DELTA-ANDROSTEN-17B-OL-3-ONE
halotensin
HOMOSTERONE
oreton
ORQUISTERON
PRIMOTESTON
testex
testoderm
TESTOSTERON
TESTOSTERONE | [EINECS(EC#)]
200-370-5 | [Molecular Formula]
C19H28O2 | [MDL Number]
MFCD00003654 | [Molecular Weight]
288.42 | [MOL File]
58-22-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline odourless solid | [Melting point ]
152-156 °C | [alpha ]
101 º (c=1, dioxane 25 ºC) | [Boiling point ]
370.65°C (rough estimate) | [density ]
1.0484 (rough estimate) | [refractive index ]
1.4709 (estimate) | [Fp ]
5 °C | [storage temp. ]
2-8°C
| [solubility ]
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: soluble18.2 mg/ml | [form ]
powder | [pka]
15.06±0.60(Predicted) | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents. | [Water Solubility ]
22.79mg/L(20 ºC) | [Usage]
Secreted by the testis and is converted to dihydrotestosterone in the target tissue where is appears to mediate many of the biological actions of testosterone.
CONTROLLED SUBSTANC | [Merck ]
13,9255 | [BRN ]
1915399 | [InChIKey]
MUMGGOZAMZWBJJ-DYKIIFRCSA-N | [CAS DataBase Reference]
58-22-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Testosterone(58-22-0) | [EPA Substance Registry System]
58-22-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xn | [Risk Statements ]
R60:May impair fertility.
R61:May cause harm to the unborn child.
R11:Highly Flammable.
R19:May form explosive peroxides.
R20:Harmful by inhalation.
R40:Limited evidence of a carcinogenic effect.
R63:Possible risk of harm to the unborn child.
R45:May cause cancer. | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S24/25:Avoid contact with skin and eyes .
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
UN 2252 3/PG 2
| [WGK Germany ]
3
| [RTECS ]
XA3030000
| [HS Code ]
29372900 | [Safety Profile]
Confirmed carcinogen with experimental neoplastigenic and teratogenic data. Poison by intraperitoneal route. Human teratogenic effects by unspecified route: developmental abnormalities of the urogenital system. Experimental reproductive effects. Human mutation data reported. Workers engaged in manufacture and packagmg have shown effects from this hormone, e.g., enlargement of the breasts in male workers. A promoter. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a drug for the treatment of hypogonadism and metastatic breast cancer. | [Hazardous Substances Data]
58-22-0(Hazardous Substances Data) | [Toxicity]
LD50 oral in mammal (species unspecified): > 5gm/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Manganese dioxide-->Acetylacetone-->3β,11α-Dihydroxy-5α-androstan-17-one-->Androstan-?17-?one, 3,?7-?dihydroxy-?, (3β,?5α,?7β)?--->Boldenone-->6A-HYDROXY-ANDROST-4-ENE-3,17-DIONE-->5-Androstenedione-->Androsta-1,4-diene-3,17-dione-->Epiandrosterone-->(8R,9S,10R,13S,14S,17S)-10,13-dimethylspiro[1,2,4,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthrene-3,2'-1,3-dioxolane]-17-ol-->Androst-4-en-3-one, 17-[(tetrahydro-2H-pyran-2-yl)oxy]-, (17β)--->14A-HYDROXYTESTOSTERONE--DEA*SCHEDULE II I ITEM-->Testosterone enanthate-->4-Androstenediol-->testololactone-->3-Ethoxyandrosta-3,5-dien-17β-ol-->Androstenedione | [Preparation Products]
Testosterone cypionate |
| Questions And Answer | Back Directory | [Chemical Properties]
White crystalline powder with no aroma. Its melting point is 155℃,
specific rotary power is [α]24D+109° (4%, ethanol), and its
ethanol solution has the greatest absorbance at a wavelength of
240nm. It is easily soluble in ethanol (1:5), soluble in ether
(1:100), and insoluble in water. LD50 (Large mice, venal
transfusion) 326mg/kg. Studies show that it has latent carcinogenic
effects on test animals. | [Indications and Uses]
Testosterone is the main natural male sex hormone in mammals and is
a steroid hormone with 19 carbon atoms. It is the main male sex
hormone secreted by the testes, and it is also the most active male
sex hormone. It promotes humans’ and animals’ sex organ and
secondary sex characteristic development, sperm maturation, and
protein metabolism for muscle strengthening.
Testosterone controls the growth and development of male sex organs
and male secondary sex characteristics. It is mainly used in
replacement therapy for eunuchism, treatment for male menopause
syndrome, and treatment for impotence, and it is also used in
biochemical research. | [Pharmacokinetics]
Testosterone can bind non-specifically with plasma albumins in
blood, and it can also bind with plasma sex hormone binding
globulins. It can be converted into estradiol and estrone in
peripheral tissue. Testosterone is mostly degraded in the liver,
where its A-ring is restored, and it is converted into 17-
ketosteroid under the effects of 17β-Hydroxysteroid dehydrogenase.
Along with androsterone, epiandrosterone, and etiocholanlone, it is
combined with glucuronic acid or sulfate and excreted in urine. Most
metabolites in urine that are excreted by binding to glucuronic acid
belong to 17-ketosteroids. | [Application in Particular Diseases]
In Osteoporosis:
- Testosterone replacement is not FDA approved for the prevention or treatment of osteoporosis. It should not be used solely for these indications but might be beneficial to reduce bone loss in patients needing therapy for hypogonadal symptoms. In a few studies, women receiving oral methyltestosterone 1.25 or 2.5 mg daily or testosterone implants 50 mg every 3 months had increased BMD. Various salt forms of testosterone were associated with increased BMD in some studies of hypogonadal men or senior men with normal hormone levels or mild hormonal deficiency. Transdermal gel, oral, intramuscular, and pellet testosterone products are available.
- Patients using them should be evaluated within 1 to 2 months of initiation and then every 3 to 6 months thereafter.
|
| Hazard Information | Back Directory | [Description]
Testosterone (CRM) (Item No. ISO60154) is a certified reference material categorized as an anabolic androgenic steroid.1 Testosterone is an endogenous metabolite of androstenedione (Item Nos. ISO60161 | 15874) and estradiol (Item Nos. ISO60155 | 10006315).2 Anabolic steroids, including testosterone, have been used to enhance physical performance in athletes.1 Testosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications. | [Description]
Testosterone (Item No.15645) is an analytical reference material categorized as an anabolic androgenic steroid.1 Testosterone is an endogenous metabolite of androstenedione (Item Nos. ISO60161 | 15874) and estradiol (Item Nos. ISO60155 | 10006315).2 Anabolic steroids, including testosterone, have been used to enhance physical performance in athletes.1 Testosterone is regulated as a Schedule III compound in the United States. This product is intended for research and forensic applications. | [Uses]
androgen, antineoplastic | [Uses]
Rivastigmine metabolite | [Uses]
Secreted by the testis and is converted to dihydrotestosterone in the target tissue where is appears to mediate many of the biological actions of testosterone.
CONTROLLED SUBSTANC | [Uses]
Testosterone secreted by the testis is converted to dihydrotestosterone in the target tissues where it appears to mediate many of the biological actions of testosterone. Androgens direct the development of the male phenotype during embryogenesis and at puberty. | [Uses]
Testosterone, Principal hormone of the testes, produced by the interstitial cells. Major circulating androgen; converted by 5α-reductase in androgen-dependent target tissues to 5α-dehydrotestosterone
which is required for normal male sexual differentiation. Also converted by aromatization to Estradiol.
Testerone is a controlled substance (anabolic steroid). Androgen. | [Definition]
ChEBI: An androstanoid having 17beta-hydroxy and 3-oxo groups, together with unsaturation at C-41C-5.. | [General Description]
Testosterone, 17β-hydroxyandrost-4-en-3-one, is a naturally occurring androgen in men. Inwomen, it mainly serves as a biosynthetic precursor to estradiolbut also has other hormonal effects. It is rapidly metabolizedto relatively inactive 17-ones, however,preventing significant oral activity. Testosterone is availablein a transdermal delivery system (patch), a gel formulation, abuccal system, and as implantable pellets. | [Hazard]
A confirmed carcinogen. | [Health Hazard]
Controls secondary male sex characteristics Maintains functional competence of male reproductive ducts and glands
Increases protein anabolism; maintains spermatogenesis; inhibits follotropin
Increases male sex behavior; increases closure of epiphyseal plates | [Biological Activity]
Endogenous androgen receptor agonist. | [Biochem/physiol Actions]
Testosterone secreted by the testis is converted to dihydrotestosterone in the target tissues where it appears to mediate many of the biological actions of testosterone. Androgens direct the development of the male phenotype during embryogenesis and at puberty. | [Synthesis]
Testosterone, 17|?-hydroxyandrost-4-ene-3-one (29.1.5), is made in a num�ber of ways from different substances, including cholesterol, although it is most often
made from androstenolone acetate. In order to do this, the keto-group at C17 of the steroid
system of androstenolone acetate is reduced to a hydroxyl group by either sodium boro�hydride, lithium aluminum hydride, or hydrogen over Raney nickel, all of which result in
a 17|?-hydroxy compound. In the given example, reduction by sodium borohydride or
hydrogen over Raney nickel leads to the formation of 3|?-acetoxy-5-androsten-17|?-ol
(29.1.1). The hydroxyl group resulting from reduction then undergoes acylation by ben�zoyl chloride in pyridine, which forms a diester (29.1.2). After that, taking into consider�ation the differences in the acidic region of the two ester groups present in the molecule as
well as the long-known fact that 17-hydroxy-group ester derivatives are harder to
hydrolyze than 3-hydroxy-group ester derivatives, the acetyl protection of the hydroxyl
group at C3 is removed by selective hydrolysis using potassium hydroxide in ethanol, and
the resulting alcohol (29.1.3) is oxidized to a ketone (29.1.4) by aluminum isopropylate in
the presence of cyclohexanone as a hydrogen acceptor, during which isomerization of the
double bond from position C5¨CC6 to C4¨CC5 simultaneously takes place. Subsequent hydrol�ysis of the remaining ester region of the molecule using an alkali gives the desired testos�terone (29.1.5) . When necessary to convert this into the corresponding ester
(propionate, enantate, cypionate, and a few other testosterone esters), the necessary acyla�tion can be accomplished.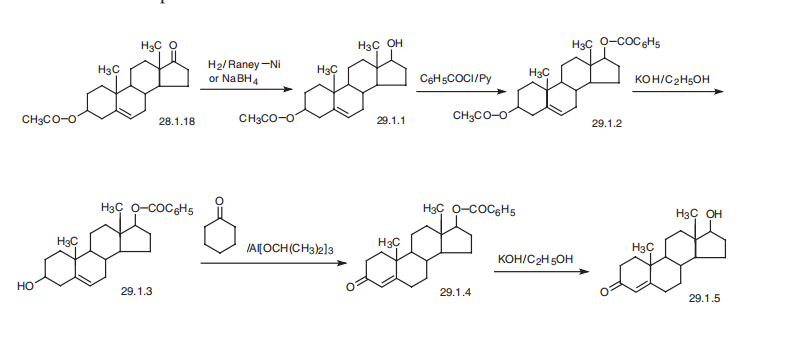
| [Purification Methods]
Crystallise testosterone from aqueous acetone, hexane or isoPrOH. The long needles that separated from EtOH/AcOH were used for X-ray crystallography [Roberts et al. J Chem Soc Perkin Trans II 1978 1973.] The acetate [1045-69-8] crystallises from MeOH or aqueous Me2CO, with m 140-141o and [] D 20 +87.8o (c 1, EtOH). [Ruzicka et al. Helv Chim Acta 18 1478 1935 and 19 99, 842 1936, Beilstein 8 IV 974.] |
|
|