| Identification | More | [Name]
Levonorgestrel | [CAS]
797-63-7 | [Synonyms]
L-NORGESTREL
13-ethyl-17-alpha-ethynyl-17-beta-hydroxy-4-gonen-3-one
13-ethyl-17-alpha-ethynylgon-4-en-17-beta-ol-3-one
13-ethyl-17-hydroxy-19-dinor-17-alpha-pregn-4-en-20-yn-3-on(+)-1
17-alpha-ethinyl-13-beta-ethyl-17-beta-hydroxy-4-estren-3-one
17-beta-hydroxy-18-methyl-19-nor-17-alpha-pregn-4-en-20-yn-3-one
17-ethynyl-18-methyl-19-nortestosteron
17-ethynyl-18-methyl-19-nortestosterone
18-methyl-17-alpha-ethynyl-19-nortestosterone
18-methylnorethindrone
follistrel
levonova
microluton
norplant2
norplantii
postinor
wy-5104
D(minus)-norgestrel
Norgestrel (L-)
13β-ethyl-17α-ethynyl-17β-hydroxygon-4-en-3-one | [EINECS(EC#)]
212-349-8 | [Molecular Formula]
C21H28O2 | [MDL Number]
MFCD06198806 | [Molecular Weight]
312.45 | [MOL File]
797-63-7.mol |
| Chemical Properties | Back Directory | [Definition]
A synthetic steroid hormone used
as an ingredient of oral contraceptives. Approved
by FDA. | [Appearance]
White Solid | [Melting point ]
206°C | [alpha ]
D20 -32.4° (c = 0.496 in CHCl3) | [Boiling point ]
392.36°C (rough estimate) | [density ]
1.0697 (rough estimate) | [refractive index ]
1.4900 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Practically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in ethanol (96 per cent). | [form ]
neat | [pka]
13.09±0.40(Predicted) | [color ]
Crystals from MeOH/CHCl3 | [optical activity]
[α]/D -28 to -36° (C= 1.0g/100 ml in CDCl3) | [Water Solubility ]
10mg/L(temperature not stated) | [Usage]
An emergency contraceptive. Levonorgestrel is safe, tolerated and effective in emergency contraception in woman | [Merck ]
13,6736 | [BCS Class]
1 | [InChI]
InChI=1/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16-,17+,18+,19-,20-,21-/s3 | [InChIKey]
WWYNJERNGUHSAO-HABXDNTMNA-N | [SMILES]
C([C@]12CC[C@]3([H])[C@@]4([H])CCC(=O)C=C4CC[C@@]3([H])[C@]1([H])CC[C@@]2(O)C#C)C |&1:1,4,6,16,18,22,r| | [CAS DataBase Reference]
797-63-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
JF8225000
| [HS Code ]
2937230000 | [Hazardous Substances Data]
797-63-7(Hazardous Substances Data) |
| Questions And Answer | Back Directory | [Overview]
Levonorgestrel[LNG] is a synthetic progestational hormone with actions similar to those of progesterone and about twice as potent as its racemic or[+-]-isomer[norgestrel][1]. It is a kind of emergency contraception, referring as a drug or device used to prevent pregnancy after unprotected sexual intercourse[including sexual assault] or after a recognized contraceptive failure[1]. It is used for contraception, control of menstrual disorders, and treatment of endometriosis. Levonorgestrel is marketed mostly as a combination oral contraceptive under several brand names such as Alesse, Triphasil, and Min-Ovral. It is also marketed under the brand of Plan B.
Levonorgestrel was discovered in 1963 and was introduced for medical use together with ethinylestradiol in 1970.[2, 3] It is listed as an essential medicine by the World Health Organization, being the most effective and safe medicines needed in a health system.[4] It is available as a generic medication.[5]
Levonorgestrel is a levo-isomer of Norgestrel. The progestins include the naturally occurring hormone progesterone, 17a-acetoxyprogesterone derivatives in the pregnane series, 19-nortestosterone derivatives(estranes), and norgestrel and related compounds in the gonane series. The 19-nortestosterone derivatives were developed for use as progestins in oral contraceptives, and while their predominant activity is progestational, they exhibit androgenic and other activities. The gonanes are a more recently developed series of “19-nor” compounds, containing an ethyl rather than a methyl substituent in the 13-position, and they have diminished androgenic activity[6].
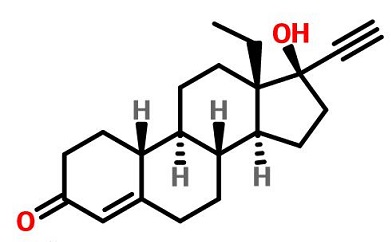
Figure 1. The chemical structure of levonorgestrel | [Indication]
Levonorgestrel is a progestin-only emergency contraceptive indicated for the prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. To obtain optimal efficacy, its should be taken as soon as possible within 72 hours of intercourse. In US, it(Plan B) is available only by prescription for women younger than age 17 years, and available over the counter for women 17 years and older. However, it should be noted that it is not indicated for routine use as a contraceptive[7].
| [Pharmacokinetics]
Progesterone is rapidly absorbed following administration by any route. Its half-life in the plasma is approximately 5 minutes, and small amounts are stored temporarily in body fat. It is almost completely metabolized in liver to pregnanediol and conjugated with glucuronic acid. It is excreted into the urine as pregnanediol glucuronide. Most of the synthetic progestins are extensively metabolized to inactive products that are excreted mainly in the urine. Route of administration of levonorgestrel is oral. Duration of action is 1-3 days. It has Androgenic, Antiestrogenic and Anabolic activities[8]. The synthetic progestins have much longer half-lives, e.g., approximately 7 hours for norethindrone, 16 hours for norgestrel, 12 hours for gestodene, and 24 hours for MPA[6].
| [Pharmacodynamics]
Levonorgestrel is a synthetic form of the naturally occurring female sex hormone, progesterone[1]. The physiological effect of progesterone is as below[1]: during woman's normal menstrual cycle, an egg matures and is released from the ovaries[ovulation]. The ovary then produces progesterone to prevent the release of further eggs and priming the lining of the womb for a possible pregnancy. If pregnancy occurs, progesterone levels in the body remain high, maintaining the womb lining. If pregnancy does not occur, progesterone levels in the body fall, resulting in a menstrual period. Levonorgestrel tricks the body processes into thinking that ovulation has already occurred, by maintaining high levels of the synthetic progesterone. This prevents the release of eggs from the ovaries.
| [Mode of action]
LNG can prevent pregnancy several different ways, depending on the cycle day of unprotected sex and the day on which treatment is initiated[9]. To be effective, postcoital treatment should inhibit or delay ovulation or inhibit implantation. If sexual intercourse takes place shortly before or at the time of ovulation, and treatment is given after ovulation, an inhibitory effect on embryo development or implantation is necessary to prevent pregnancy. How LNG prevents pregnancy in the doses used for EC is not known[10]. However, emergency contraceptives do not interrupt pregnancy after implantation[6].
Effects on the ovulatory process:
LNG, 0.75 mg, in repeated doses has been shown to suppress proliferative activity of the endometrium when administered during the follicular phase. It suppresses ovarian function when administered in the late follicular phase and around the time of ovulation. No significant endometrial changes were detected when LNG was administered in the secretory phase. However, ovulation process is the main target for emergency contraception[10].
Effects on the endometrium:
High dose ethinyl estradiol –norgestrel ECPs effectively suppresses progesterone associated endometrial protein in the midluteal uterus, potentially altering the endometrial environment unfavorably and affecting the survival of the early embryo[20]. LNG existing evidence does not support the hypothesis that it alters endometrial receptivity or impedes implantation. From a physiological and pharmacological point of view, the administration of synthetic progestogens such as LNG is highly unlikely to reduce endometrial receptivity. Progestogens, whether natural or synthetic, are so called because of their ability to sustain pregnancy in ovariectomised animals[11]. The 25% failure rate of EC and the fact that it works best when used soon after sexual intercourse[12] are further reasons for doubting that this method impedes pregnancy by interference with postfertilisation events.
Effects on the migration and function of spermatozoa:
LNG has some experimental evidence for transportation of spermatozoa from the vagina to the fallopian tube. Administration of 400 μg LNG(57% of the current LNG dosage) 3–10 hours after sexual intercourse affected sperm migration between 3 and 9 hours after treatment. It reduced the number of spermatozoa recovered from the uterine cavity, increased the pH of the uterine fluid(which immobilized spermatozoa), and increased the viscosity of cervical mucus(which impeded further passage of sperm cells into the uterine cavity)[13]. These results are highly relevant to the actions of LNG used as an EC.
| [Adverse reactions]
Side effects are medically minor but troublesome to the patients. No deaths or serious complications have been causally linked to LNG.
Nausea and vomiting: The main side effects are gastrointestinal. The Yuzpe regimen causes nausea in up to 50% of recipients and as many as 19% experience vomiting. Since the pills are completely absorbed within one hour, replacement dosing is unnecessary if vomiting occurs after this time. The LNG regimen is significantly better tolerated, with nausea in 23% and vomiting 6%[14]. Meclizine is effective for preventing nausea and vomiting associated with the Yuzpe regimen of LNG[15].
Other common side effects with LNG regimens are dizziness, fatigue, headache, breast tenderness, lower abdominal pain and menstrual irregularities[14]. Although some women may experience spotting, the majority of women have their menstrual period on time or slightly early[16].
Risk of Ectopic pregnancy: Camp S.L. et al. has calculated from various clinical trials of 0.75 mg tablets of LNG used for EC(including almost 6000 women), 98 pregnancies were reported; 1 of which was ectopic, giving an ectopic pregnancy rate of 1%[9], i.e. we cannot deny a risk of ectopic pregnancy.
Teratogenic Effects: The limited data on teratogenic effects come from a relatively small number of reports in which treatment was not successful, and the woman elected to continue the pregnancy. No evidence exists of a specific syndrome of anomalies or an apparent increase in the incidence of anomalies. It is important to recognize that no studies have investigated teratogenic effects associated with the use of oral emergency contraception[17].
| [Precautions/contraindication]
Women do not need provider intervention to use the LNG regimen of ECPs safely and effectively[18]. Progestogens only can be started in healthy non-pregnant women without screening procedures. According to WHO international guidelines, the minimum requirements before starting contraception with a combined estrogen -progestogens product consists of asking for a personal and family history of deep vein thrombosis and measuring blood pressure at baseline and follow-up. Combined agents are best avoided by women over 35 years who smoke[19]. If normal menstrual bleeding does not occur within 21 days after taking LNG or by 28 days if an oral contraceptive was initiated after LNG use, a pregnancy test should be taken[20].
Levonorgestrel is safe—even if taken in pregnancy, evidence suggests that it will not harm the pregnancy or the fetus —and it has no potential for addiction. The regimen has few contraindications, all of which should be easy for the consumer to identify for herself without needing an examination[21]. There are only three contraindications to the use of LNG as EC: existing pregnancy, undiagnosed vaginal bleeding or a known allergy to any ingredient in the product[9]. There are no absolute medical contraindications to the use of emergency contraception with the exception of pregnancy, and this is only because it is ineffective. According to WHO there are no known medical contraindications to the use of hormonal EC, aside from allergy to one of the constituents[22].
| [References]
- https://www.drugbank.ca/drugs/DB00367
- https://books.google.com/books?id=ja2nBgAAQBAJ&pg=PA383
- https://books.google.com/books?id=FsKpBAAAQBAJ&pg=PA77
- http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1
- https://books.google.ca/books?id=BwqEAgAAQBAJ&pg=PA310
- David SL, George MS. Estrogens and Progestins. In: Laurence L. Brunton, John S. Lazo, Keith L. Parker Goodman & Gilman’s the pharmacological basis of therapeutics – 11th edition. New York: McGraw-Hill Medical Publishing Division; 2006. 1541-1571.
- https://www.rxlist.com/plan-b-drug.htm#description
- Chrousos GP. The Gonadal Hormones and Inhibitors. In: Katzung BG. Editors. Basic and Clinical Pharmacology. 9th International edition, Boston, New York, New Delhi, McGrawHill Companies Inc 2004: 661-692.
- Camp SL, Wilkersona DS, Raine TR. The benefits and risks of over-the-counter availability of Levonorgestrel emergency contraception. Contraception 2003; 68: 309–317.
- Marions L, Hultenby K, Lindell I, Sun X, Stabi B, Danielsson KG. Emergency Contraception with Mifepristone and Levonorgestrel: Mechanism of Action. Obstet Gynecol 2002; 100[1]:65–71
- Croxatto HB. Emergency contraception pills: how do they work? IPPF Medical Bulletin 2002; 36[6]: 1-2.
- Piaggio G, Von Hertzen H, Van Look PFA. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet 1999; 353: 721.
- Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of dlnorgestrel inpostcoital contraception. Contraception 1974; 10:411-24.
- Task Force on Postovulatory Methods of Fertility Regulation. Randomized controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet 1998; 352: 428–33.
- Raymond EG, Creinin MD, Barnhart KT, Lovvorn AE, Rountree RW, Trussell J. Meclizine for Prevention of Nausea Associated With Use of Emergency Contraceptive Pills: A Randomized Trial. Obstet Gynecol 2000; 95[2]: 271–277.
- Glasier A,Thong KJ, et al. Mifepristone[RU 486] compared with high dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041-4.
- Weismiller DG. Emergency Contraception. Am Fam Physician 2004; 70[4]:707-14.
- Raymond EG, Chen PL, Dalebout SM. “Actual Use” Study of Emergency Contraceptive Pills Provided in a Simulated Over-the-Counter Manner. Obstet Gynecol 2003; 102[1]:17–23.
- Amy JJ, Tripathi V. Contraception for women: an evidence based overview. BMJ 2009; 339: b 2895.
- Kleppin S. Levonorgesterel for Emergency Contraception: Controversy continues regarding Plan B. JPSW 2006; 21-23
- Raymond EG, Dalebout SM, Camp SI. Comprehension of a Prototype Over-the-Counter Label for an Emergency Contraceptive Pill Product. Obstet Gynecol 2002; 100[2]:342–9.
- Dunn S, Guilbert E. Emergency Contraception. J Obstet Gynaecol Can 2003; 25[8]:673–9.
|
| Hazard Information | Back Directory | [Description]
Levonorgestrel exhibits some androgenic activity but no glucocortic oid or antimineraloc orticoid action. Levonorgestrel can be administered orally, transdermally (combined with estradiol and formulated as a 7-day patch), and for prolonged, continuous use, via an intrauterine device (IUD). The oral bioavailability of levonorgestrel is approximately 95%. From a protein binding pers pective, 48% of an oral dose is bound to SHBG, and 50% is bound to albumin. Levonorgestrel under goes metabolic reduction of its ketone and is hydroxylated. | [Chemical Properties]
White Solid | [Originator]
Ovrette,Wyeth,US,1968 | [Uses]
An emergency contraceptive. Levonorgestrel is safe, tolerated and effective in emergency contraception in woman | [Uses]
progestin | [Manufacturing Process]
To 0.7 gram of (+/-)-1,4-dihydro-17α-ethynyl-18-homo-oestradiol 3-methyl ether in 36 cc methanol was added 1.6 cc water and 2.4 cc concentrated hydrochloric acid. After standing at room temperature for 2 hours ether was added, and the washed and dried ethereal solution was evaporated, yielding a gum which was dissolved in 5 cc benzene and the solution absorbed on 50 grams of an activated fuller's earth. Elution with light petroleum containing increasing proportions of benzene gave a crystalline by-product: further elution with benzene containing a small proportion of ether gave a crystalline product which was recrystallized from ethyl acetate, yielding 0.11 gram of (+/-)-17α-ethynyl-18-homo-19-nortestosterone. MP 203° to 206°C.
| [Brand name]
Mirena (Berlex);
Norplant (Wyeth); Plan B (Duramed). | [Therapeutic Function]
Progestin | [Safety Profile]
Human reproductive effects byingestion: menstrual cycle changes, fertility index.Questionable carcinogen with experimental neoplastigenicdata. When heated to decomposition it emits acrid smokeand irritating fumes. |
|
|