| Identification | More | [Name]
Lithium hydride | [CAS]
7580-67-8 | [Synonyms]
LITHIUM HYDRIDE
Hydrure de lithium
hydruredelithium
LiH
Lithium hydride (LiH)
lithiumhydride(lih)
lithiumhydride,fusedsolid
lithiummonohydride
Lithiumhydrideminmeshgraypowder
Lithiumhydridetech
LITHIUM HYDRIDE 98% &
LITHIUM HYDRIDE, POWDER,-30 MESH, 95%
LITHIUM HYDRIDE, PIECES
LITHIUM HYDRIDE, POWDER
Lithium hydride, in resealable cans, pure, 98%
Lithium hydride, pure, 98%
Lithiumhydride,min.95%
LITHIUM HYDRIDE ,-30 MESH
Lithium hydride, 99.4% (metals basis)
Lithium hydride, 98%, pure | [EINECS(EC#)]
231-484-3 | [Molecular Formula]
HLi | [MDL Number]
MFCD00011074 | [Molecular Weight]
7.95 | [MOL File]
7580-67-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Lithium hydride is an off-white to grayish,
translucent, odorless solid or white powder that darkens
rapidly on exposure to light. | [Melting point ]
680 °C(lit.)
| [bulk density]
290-430kg/m3 | [density ]
0.82 g/mL at 25 °C(lit.)
| [storage temp. ]
water-free area | [solubility ]
Slightly soluble in dimethylformamide. Insoluble in acetone, benzene and toluene. | [form ]
powder
| [color ]
White to gray | [Odor]
odorless | [PH]
>7 (21g/l, H2O, 20℃) | [Water Solubility ]
reacts | [Sensitive ]
Air & Moisture Sensitive | [Merck ]
14,5533 | [Exposure limits]
TLV-TWA 0.025 mg/m3 (ACGIH). | [InChI]
InChI=1S/Li.H | [InChIKey]
SIAPCJWMELPYOE-UHFFFAOYSA-N | [SMILES]
[LiH] | [CAS DataBase Reference]
7580-67-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Lithium hydride(7580-67-8) | [EPA Substance Registry System]
7580-67-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,C,T | [Risk Statements ]
R14:Reacts violently with water.
R34:Causes burns.
R25:Toxic if swallowed.
R14/15:Reacts violently with water, liberating extremely flammable gases . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S7/8:Keep container tightly closed and dry .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S25:Avoid contact with eyes . | [OEB]
D | [OEL]
TWA: 0.025 mg/m3 | [RIDADR ]
UN 1414 4.3/PG 1
| [WGK Germany ]
2
| [RTECS ]
OJ6300000
| [F ]
10 | [TSCA ]
Yes | [HazardClass ]
4.3 | [PackingGroup ]
I | [HS Code ]
28500090 | [Safety Profile]
Poison by inhalation. A
severe eye, skin, and mucous membrane
irritant. Upon contact with moisture, lithium
hydroxide is formed. The LiOH formed is
very caustic and therefore highly toxic,
particularly to lungs and respiratory tract,
skin, and mucous membranes. The powder
ignttes spontaneously in air. The solid can
ignite spontaneously in moist air. Mixtures
of the powder with liquid oxygen are
explosive. Ignttes on contact with dinitrogen oxide, oxygen + moisture. To fight fire, use
special mixtures of dry chemical. See also
LITHIUM COMPOUNDS and
HYDRIDES. | [Hazardous Substances Data]
7580-67-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 78 mg/kg | [IDLA]
0.5 mg/m3 |
| Hazard Information | Back Directory | [General Description]
A white or translucent crystalline mass or powder. The commercial product is light bluish-gray lumps due to the presence of minute amounts of colloidally dispersed lithium. | [Reactivity Profile]
LITHIUM HYDRIDE(7580-67-8) is a strong reducing agent. May decompose violently in contact with most oxidizing materials. Reacts exothermically with water to form caustic lithium hydroxide and hydrogen gas; the hydrogen may ignite. May ignite spontaneously in moist air. Mixtures with liquid oxygen are explosive. Ignites on contact with dinitrogen oxide [Mellor, 1967, vol. 8, suppl. 2.2, p. 214]. | [Air & Water Reactions]
Burns readily in air, particularly if powdered. May ignite spontaneously in moist air. Reacts rapidly with water to form caustic lithium hydroxide and hydrogen [Bretherick 1979 p. 107]. | [Health Hazard]
This material is relatively toxic to people. It is more likely to cause irritation of skin and mucous membrane tissues rather than death. Its effects are primarily acute. A massive exposure to the eyes and by inhalation may be lethal. Those experiencing any ailment of the upper respiratory tract (e.g., bronchitis or pneumonia) are at a greater risk. | [Potential Exposure]
Lithium hydride is used in preparation
of lithium aluminum hydride; as a desiccant; it is used in
hydrogen generators and in organic synthesis as a reducing
agent and condensing agent with ketones and acid esters; it
is reportedly used in thermonuclear weapons. | [Fire Hazard]
In a fire, irritating alkali fumes may form. Lithium hydride can form airborne dust clouds which may explode on contact with flame, heat, or oxidizing materials. Additionally, spontaneous ignition occurs when nitrous oxide and lithium hydride are mixed. Lithium hydride also forms explosive mixtures with liquid oxygen. Contact with heat, moisture or acid causes exothermic reaction and evolution of hydrogen as well as lithium hydroxide. Incompatible with air and moisture, nitrous oxide, strong oxidizers, and liquid oxygen. Lithium hydride may ignite spontaneously in air and should be maintained and handled out of contact with air and moisture. Any contact with nitrous oxide; airborne powders may ignite upon reaching moisture. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water or
milk. Do not induce vomiting. Medical observation is
recommended for 24 to 48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for
pulmonary edema, a doctor or authorized paramedic may
consider administering a drug or other inhalation therapy. | [Shipping]
UN1414 Lithium, Hazard Class: 4.3; Labels:
4.3-Dangerous when wet material. UN2805 Lithium
hydride, fused solid, Hazard Class: 4.3; Labels: 4.3-
Dangerous when wet material | [Incompatibilities]
A Strong reducing agent. Incompatible
with oxidizers, halogenated hydrocarbons; acids can cause
fire and explosion. Reacts with water, forming caustic lithium hydroxide and flammable hydrogen gas; reaction may
cause ignition. May ignite spontaneously in moist air and
may reignite after fire is extinguished. Dangerous when
wet. Reacts with water to form hydrogen and lithium
hydroxide. Powdered form and liquid oxygen form an
explosive compound. Decomposes exothermically on contact with acids and upon heating to about 500�C, producing
flammable hydrogen gas. Reacts with carboxylic acids,
lower alcohols; chlorine, and ammonia (at 400�C), forming
explosive hydrogen gas. | [Description]
Lithium hydride is an off-white to grayish,translucent, odorless solid or white powder that darkens rapidly on exposure to light. Molecular weight = 7.95; Specificgravity (H2O:1)=0.78; Boiling point = 850℃ (decomposesbelow BP); Freezing/Melting point = 689℃; Autoignitiontemperature = 200℃. Hazard Identification (based onNFPA-704 M Rating System): Health 3, Flammability 4,Reactivity 2. A combustible solid that can form airbornedust clouds which may explode on contact with flame, heat,or oxidizers. | [Chemical Properties]
Lithium hydride (LiH) is a crystalline salt substance(face-centered cubic) that is white in its pure form, As an engineering material, it has properties of interest in many technologies. For example,the high hydrogen content and light weight of LiH make it useful for neutron shields and moderators in nuclear power plants. In addition, the high heat of fusion combined with light weight make LiH appropriate for heat storage media for solar power plants on satellites and may be used as a heat sink for different applications. Typically, processes for production of LiH involve handling of LiH at temperatures above its meltingpoint (688 DC). Type 304L stainless steel is utilized for many process components handling molten LiH.
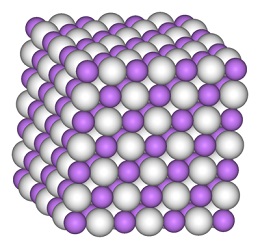
Lithium hydride is a typical ionic hydride with lithium cations and hydride anions. Electrolysis of molten material results in formation of lithium metal at the cathode and hydrogen at the anode. The lithium hydride-water reaction, which results in the release of hydrogen gas, is also indicative of a negatively charged hydrogen.
| [Chemical Properties]
Lithium hydride is an off-white to grayish,
translucent, odorless solid or white powder that darkens
rapidly on exposure to light. | [Waste Disposal]
Lithium hydride may be
mixed with sand, sprayed with butanol and then with water,
neutralized and flushed to a sewer with water | [Physical properties]
White crystalline solid; cubic crystals; density 0.82 g/cm3; melts at 686.4°C; decomposes in water; soluble in acids. | [Definition]
lithium hydride: A white solid,LiH; cubic; r.d. 0.82; m.p. 680°C; decomposesat about 850°C. It is producedby direct combination of theelements at temperatures above500°C. The bonding in lithium hydrideis believed to be largely ionic;i.e. Li+H- as supported by the factthat hydrogen is released from theanode on electrolysis of the moltensalt. The compound reacts violentlyand exothermically with water toyield hydrogen and lithium hydroxide.It is used as a reducing agent toprepare other hydrides and the 2Hisotopic compound, lithiumdeuteride, is particularly valuable fordeuterating a range of organic compounds.Lithium hydride has alsobeen used as a shielding material forthermal neutrons. | [reaction suitability]
reagent type: reductant | [storage]
The product should be handled under an inert atmosphere to avoid contamination and a fire. Powdered lithium hydride burns readily when exposed to the air. However, large pieces of the material are less flammable. Lithium hydride, like other strong bases, is harmful to the skin and should be handled with caution. | [Purification Methods]
It should be a white powder; otherwise replace it. It darkens rapidly on exposure to air and is decomposed by H2O to give H2 and LiOH, and reacts with lower alcohols. One gram in H2O liberates 2.8L of H2 (could be explosive). [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963.] |
| Questions and Answers (Q&A) | Back Directory | [Uses]
Industry
Application
Role/benefit
Hydrogen
Hydrogen storage
Storage material/has the highest hydrogen content of any hydride
Hydrogen preparation
Hydrogen source/reacts violently with water to yield hydrogen
Nuclear
Nuclear reactors
Neutron shield material
Thermonuclear weapons
Fusion fuel (lithium-6 deuteride)
Astronomy
Rocket fuel
Excellent thermal value
Organic chemistry
Synthesis of complex metal hydrides
Raw material
Preparation of LiAlH4,LiBH4 and LiBHET3 ,etc.
Preparation of other hydrides amides and 2H isotopic compound
Reducing agent
| [Preparation]
Lithium hydride is prepared by heating lithium metal with hydrogen above 440°C. The reaction is exothermic and can be controlled once it is initiated, without external heating. The heat of formation is greater than that of sodium hydride:
2Li + H2 → 2LiH
| [Reactions]
Lithium hydride reacts vigorously with water, forming lithium hydroxide with the evolution of hydrogen:
LiH + H2O → LiOH + H2
The hydride also reacts with ammonia forming lithium amide and evolving hydrogen:
LiH + NH3 → LiNH2 + H2
Lithium hydride is a strong reducing agent and would, therefore, react with compounds that contain oxygen. Even many highly stable oxides of metals and nonmetals can be reduced. It reduces metal oxides to metals and carbon dioxide to carbon:
Fe3O4 + 4LiH → 3Fe + 4NaOH
2LiH + CO2 → Li2O + C + H2O
It undergoes violent reactions with oxidizing agents.
Lithium hydride reacts with aluminum hydride forming lithium aluminum hydride, a powerful reducing agent:
LiH + AlH3 → LiAlH4
Lithium hydride consisting of Li+ and H– ions exhibits properties of an ionic salt, both cationic and anionic; such as a strong electrolyte. Thus, when electrolyzed at temperatures slightly below its melting point, it dissociates to Li+ and H¯ ions. Hydrogen gas is liberated at the anode.
The hydride ion, H:¯ being a strong base, would react with alcohols, forming alkoxides and liberating hydrogen:
CH3CH2OH + LiH → CH3CH2OLi + H2
(ethanol) (lithium ethoxide)
(CH3)3COH + LiH → (CH3)3COLi + H2
(tert-butanol) (lithium tert-butoxide)
|
|
|