| Identification | More | [Name]
Triphenylphosphine | [CAS]
603-35-0 | [Synonyms]
'LGC' (4006)
PHOSPHORUSTRIPHENYL
PP-360
TPP
TRIPHENYL PHOSPHOROUS
TRIPHENYLPHOSPHORUS
TRP
phosphine,triphenyl
Phosphine,triphenyl-
Phosphoris triphenyl
Trifenylfosfin
Triphenylphosphane
triphenyl-phosphane
Triphenylphosphide
triphenyl-phosphin
Triphenyphosphine
Dehydrocholic acid CP2000
Trihenylphosphine
TRIPHENYLPHOSPHINE, REAGENTPLUS, 99%
TRIPHENYLPHOSPHINE REAGENTPLUS(TM) 99% | [EINECS(EC#)]
210-036-0 | [Molecular Formula]
C18H15P | [MDL Number]
MFCD00003043 | [Molecular Weight]
262.29 | [MOL File]
603-35-0.mol |
| Chemical Properties | Back Directory | [Appearance]
White, crystalline solid. Insoluble inwater; slightly soluble in alcohol;
soluble in benzene, acetone, carbon tetrachloride.
Combustible. | [Melting point ]
79-81 °C(lit.)
| [Boiling point ]
377 °C(lit.)
| [bulk density]
500-600kg/m3 | [density ]
1.132 | [vapor density ]
9 (vs air)
| [vapor pressure ]
5 mm Hg ( 20 °C)
| [refractive index ]
1.6358 | [Fp ]
181 °C
| [storage temp. ]
Store at RT. | [solubility ]
water: soluble0.00017 g/L at 22°C | [form ]
Crystals, Crystalline Powder or Flakes | [color ]
White | [Specific Gravity]
1.132 | [Odor]
odorless | [Stability:]
Stable. Incompatible with oxidizing agents, acids. | [Water Solubility ]
Insoluble | [Hydrolytic Sensitivity]
8: reacts rapidly with moisture, water, protic solvents | [Detection Methods]
GC | [Merck ]
14,9743 | [BRN ]
610776 | [InChIKey]
RIOQSEWOXXDEQQ-UHFFFAOYSA-N | [CAS DataBase Reference]
603-35-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Phosphine, triphenyl-(603-35-0) | [EPA Substance Registry System]
603-35-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N | [Risk Statements ]
R22:Harmful if swallowed.
R43:May cause sensitization by skin contact.
R53:May cause long-term adverse effects in the aquatic environment.
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R48/20/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation and if swallowed . | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
3077 | [WGK Germany ]
2
| [RTECS ]
SZ3500000
| [F ]
9 | [Autoignition Temperature]
425 °C | [TSCA ]
Yes | [HS Code ]
29310095 | [Safety Profile]
Moderately toxic by
ingestion. Mildly toxic by inhalation. A skin
and eye irritant. Combustible when exposed
to heat or flame. Slight explosion hazard in
the form of vapor when exposed to flame.
Can react vigorously with oxidizing
materials. To fight fire, use dry chemical,
fog, CO2. When heated to decomposition it
emits highly toxic fumes of phosphne and
POx. See also PHOSPHINE and
PHENOL. | [Hazardous Substances Data]
603-35-0(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 700 mg/kg LD50 dermal Rabbit > 4000 mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Hydrochloric acid-->Sodium-->Phosphorus trichloride-->Bromobenzene-->Triphenyl phosphite | [Preparation Products]
6-AMINO-CHROMEN-2-ONE-->5-AMINOMETHYL-PYRROLIDIN-2-ONE-->N10-(TRIFLUOROACETYL)PTEROIC ACID-->Cefixime-->2-[2-(DIPHENYLPHOSPHINO)ETHYL]PYRIDINE-->2-(DIPHENYLPHOSPHINO)ETHYLAMINE-->5-[(1,3-DIOXO-1,3-DIHYDRO-2H-ISOINDOL-2-YL)METHYL]-2-FURALDEHYDE-->Zidovudine-->5-Phenylthiophene-2-carboxylic acid-->2-BROMO-1,1-BINAPHTHYL-->DESACETYLVINBLASTINEAMIDE-->Tris(triphenylphosphine)ruthenium(II) chloride-->4-((1-METHYLPIPERIDIN-3-YL)METHOXY)-3,5-DICHLOROBENZENAMINE-->2-DIPHENYLPHOSPHINO-6-METHYLPYRIDINE-->1-METHYL-PROP-2-YNYLAMINE-->trans-2-Octen-1-ol-->5,5'-DIMETHYL-2,2'-DIPYRIDYL-->2,2'-DIBROMO-1,1'-BINAPHTHYL-->Aminoprofen-->3-Bromoisonicotinic acid-->BUT-3-YN-1-AMINE-->2,7-DIBROMO-9H-CARBAZOLE-->5-PHENYL-2-THIOPHENECARBALDEHYDE-->PENTYLTRIPHENYLPHOSPHONIUM BROMIDE-->TRIPHENYLPHOSPHINE DIBROMIDE-->DOXEPIN-->Bis(triphenylphosphine)nickel(II)chloride-->3-BROMOPYRIDINE-2-CARBOXYLIC ACID-->Lacidipine-->Acrivastine-->CIS-9-TETRADECENYL ACETATE-->[1,2-Bis(diphenylphosphino)ethane]dichloropalladium(II)-->6-(BOC-AMINO)-HEXYL BROMIDE-->Isotretinoin-->3-Phenyl-1H-pyrazole-->S-2-Benzothiazolyl 2-amino-alpha-(methoxyimino)-4-thiazolethiolacetate-->(Methoxymethyl)triphenylphosphonium chloride-->Ethyltriphenylphosphonium acetate-->Ivermectin-->Methyltriphenylphosphonium bromide |
| Hazard Information | Back Directory | [General Description]
White crystals. | [Reactivity Profile]
TRIPHENYL PHOSPHINE(603-35-0) reacts vigorously with oxidizing materials. . | [Air & Water Reactions]
Highly flammable. Insoluble in water. | [Health Hazard]
ACUTE/CHRONIC HAZARDS: Toxic; when heated to decomposition, emits highly toxic fumes of phosphine and POx. | [Fire Hazard]
This compound is combustible. | [Description]
Triphenylphosphine: a member of tertiary phosphines
Triphenylphosphine (TPP) is a member of tertiary phosphines, which is phosphane, in which the three hydrogens are replaced by phenyl groups. It has a role as a reducing agent and an NMR chemical shift reference compound. It is a crucial ligand utilized in the Wittig reaction for alkene synthesis. This reaction involves the formation of alkyliden-etriphenylphosphoranes from the action of butyllithium or another base on the quarternary halide. Triphenylphosphine is used to synthesise organic compounds due to its nucleophilicity and reducing character. | [Definition]
ChEBI: Triphenylphosphine is a member of the class of tertiary phosphines that is phosphane in which the three hydrogens are replaced by phenyl groups. It has a role as a reducing agent. It is a member of benzenes and a tertiary phosphine. | [Synthesis Reference(s)]
Tetrahedron Letters, 35, p. 625, 1994 DOI: 10.1016/S0040-4039(00)75855-2 | [Flammability and Explosibility]
Notclassified | [reaction suitability]
reagent type: reductant | [Purification Methods]
It crystallises from hexane, MeOH, diethyl ether, CH2Cl2/hexane or 95% EtOH. Dry it at 65o/<1mm over CaSO4 or P2O5. Chromatograph it through alumina using (4:1) *benzene/CHCl3 as eluent. [Blau & Espenson et al. J Am Chem Soc 108 1962 1986, Buchanan et al. J Am Chem Soc 108 1537 1986, Randolph & Wrighton J Am Chem Soc 108 3366 1986, Asali et al. J Am Chem Soc 109 5386 1987.] It has also been crystallised twice from pet ether and 5 times from Et2O/EtOH to give m 80.5o. Alternatively, dissolve it in conc HCl, and upon dilution with H2O it separates because it is weakly basic, it is then crystallised from EtOH/Et2O. It recrystallises unchanged from AcOH. [Forward et al. J Chem Soc Suppl. p121 1949, Muller et al. J Am Chem Soc 78 3557 1956.] 3Ph3P.4HCl crystallises out when HCl gas is bubbled through an Et2O solution, it has m 70-73o, but recrystallises very slowly and is deliquescent. The hydriodide, made by adding Ph3P to hydriodic acid, is not hygroscopic and decomposes at ~100o. The chlorate (1:1) salt has m 165-167o, but decomposes slowly at 100o. All salts hydrolyse in H2O to give Ph3P [IR, UV: Sheldon & Tyree J Am Chem Soc 80 2117 1958, pK: Henderson & Streuli J Am Chem Soc 82 5791 1960, Kosolapoff, Organophosphorus Compounds, Wiley 1950]. [Beilstein 16 IV 951.] § Available commercially on a polystyrene or polyethyleneglycol support. |
| Questions And Answer | Back Directory | [Chemical properties]
Triphenylphosphine is a common organophosphorus compound with the formula P(C6H5)3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless to pale yellow monoclinic crystals at room temperature. It is a colorless to pale yellow transparent oily liquid above the room temperature with skin irritation and a pungent odour. It dissolves in non-polar organic solvents such as benzene and diethyl ether. | [Name Reactions]
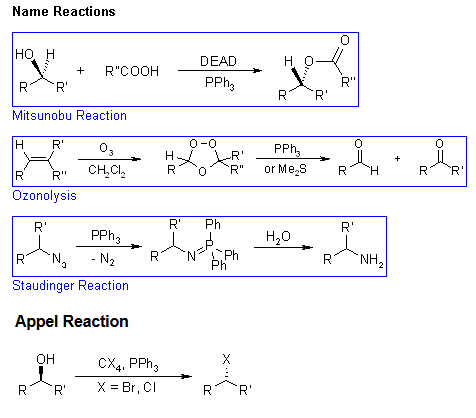
- Mitsunobu reactions
The triphenylphosphine combines with DEAD to generate a phosphonium intermediate that binds to the alcohol oxygen, activating it as a leaving group. Substitution by the carboxylate, mercaptyl, or other nucleophile completes the process.
- Ozonolysis reactions
Ozonolysis allows the cleavage of alkene double bonds by reaction with ozone. Depending on the work up, different products may be isolated: reductive work-up gives either alcohols or carbonyl compounds, while oxidative work-up leads to carboxylic acids or ketones.
- Staudinger reactions
Triphenylphosphine reacts with the azide to generate a phosphazide, which loses N2 to form an iminophosphorane. Aqueous work up leads to the amine and the very stable phosphine oxide.
- Appel reactions
The reaction of triphenylphosphine and tetrahalomethanes (CCl4, CBr4) with alcohols is a ready method to convert an alcohol to the corresponding alkyl halide under mild conditions. The yields are normally high.
This reaction is somewhat similar to the Mitsunobu Reaction, where the combination of a phosphine, a diazo compound as a coupling reagent, and a nucleophile are used to invert the stereochemistry of an alcohol or displace it.
| [Uses]
Triphenylphosphine is first sulfonated with oleum to form the trisulfonic acid.
Triphenylphosphine can be used in Wittig synthesis. It is a standard ligand in homogeneous catalysis.
Triphenylphosphine is used in the synthesis of an organophosphorus intermediate, trimethyl phosphite in ester exchange method. And then a series of organophosphorus pesticides such as dichlorvos, monocrotophos and phosphamidon can be further obtained.
In addition, it can be used as stabilizers in the synthesis of rubber and resins, antioxidants in polyvinyl chloride, and raw material in the synthesis of alkyd resins and polyester resins. | [Production methods]
In this preparation method, phenol and phosphorus trichloride was used as raw materials. After esterification and vacuum distillation, the product namely triphenyl phosphite can be obtained.
3C6H5OH + PCl3 [15~20 ℃] → (C3H5O) 3P + 3HCl
Specific process can be classified into batch and continuous processes.
(1) Batch process
The phenol was added into the reactor, after warming to melt phosphorus trichloride was added to react with phenol at 70~90 ℃. After the phosphorus trichloride addition was completed, the temperature of reaction mixture was raised to about 150 ℃. After the removal of hydrogen chloride and unreacted phenol dissolved under reduced pressure at a high temperature, the product can be achieved.
(2) The use of a tower reactor
Phenol was feeding under the condenser located in the upper portion of the tower, while phosphorus trichloride enters above the receptacle located in the lower portion of the tower. Both reacted in the tower, and the product was collected in the receiver, meanwhile by-product hydrogen chloride was introduced into the absorber tower via the upper end of the condenser. After some process of the crude ester such as distillation, the product can be obtained. |
|
|