| Identification | More | [Name]
Nicotinic acid | [CAS]
59-67-6 | [Synonyms]
3-CARBOXYPYRIDINE
3-PICOLINIC ACID
3-PYRIDINECARBOXYLIC ACID
ACIDUM-NICOTINICUM
AKOS BBS-00003719
BETA-PICOLINIC ACID
NIACIN
NICONACID
NICOTINIC ACID
PELLAGRA PREVENTIVE FACTOR
PYRIDINE-3-CARBOXYLATE
PYRIDINE-3-CARBOXYLIC ACID
PYRIDINE-BETA-CARBOXYLIC ACID
RARECHEM AL BO 0217
TIMTEC-BB SBB004279
VITAMIN B
VITAMIN B3
3-Carboxylpyridine
Acide nicotinique
acidenicotinique | [EINECS(EC#)]
200-441-0 | [Molecular Formula]
C6H5NO2 | [MDL Number]
MFCD00006391 | [Molecular Weight]
123.11 | [MOL File]
59-67-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White powder | [Melting point ]
236-239 °C(lit.)
| [Boiling point ]
260C | [bulk density]
330kg/m3 | [density ]
1.473 | [refractive index ]
1.5423 (estimate) | [Fp ]
193°C | [storage temp. ]
0-6°C | [solubility ]
18g/l | [form ]
Powder | [pka]
4.85(at 25℃) | [color ]
White to off-white | [Odor]
odorless to sl. odor, sour taste | [PH]
2.7 (18g/l, H2O, 20℃) | [Stability:]
Stable. Incompatible with strong oxidizing agents. May be light sensitive. | [biological source]
synthetic (organic) | [Water Solubility ]
1-5 g/100 mL at 17 ºC | [Usage]
Niacin feed grade is used as vitamin for poultry, swines, ruminants, fish, dogs and cats, etc. It is also used as intermediate for nicotinic acid derivatives and technical applications. WWW Link | [Detection Methods]
T,NMR | [Merck ]
14,6525 | [BRN ]
109591 | [BCS Class]
3 | [InChIKey]
PVNIIMVLHYAWGP-UHFFFAOYSA-N | [LogP]
0.360 | [Uses]
niacin is also known as vitamin B3. It is a water-soluble conditioning agent that improves rough, dry, or flaky skin, helping smooth the skin and improve its suppleness. niacin enhances the appearance and feel of hair, by increasing body, suppleness, or sheen, or by improving the texture of hair that has been damaged physically or by chemical treatment. When used in the formulation of skin care products, niacinamide and niacin enhance the appearance of dry or damaged skin by reducing flaking and restoring suppleness. | [CAS DataBase Reference]
59-67-6(CAS DataBase Reference) | [NIST Chemistry Reference]
Niacin(59-67-6) | [Storage Precautions]
Light sensitive | [EPA Substance Registry System]
59-67-6(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
1
| [RTECS ]
QT0525000
| [F ]
8 | [Autoignition Temperature]
>365 °C Dust | [TSCA ]
Yes | [HS Code ]
29362990 | [Safety Profile]
Poison by
intraperitoneal route. Moderately toxic by
ingestion, intravenous, and subcutaneous
routes. Human systemic effects: change in
clotting factors, changes in platelet count.
Questionable carcinogen with experimental
carcinogenic data. When heated to
decomposition it emits toxic fumes of NOx. | [Hazardous Substances Data]
59-67-6(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
Odorless white crystalline powder with a feebly acid taste. pH (saturated aqueous solution) 2.7. pH (1.3% solution) 3-3.5. | [Reactivity Profile]
NICOTINIC ACID(59-67-6) is incompatible with strong oxidizers. NICOTINIC ACID(59-67-6) is also incompatible with sodium nitrite. | [Air & Water Reactions]
Water soluble. | [Fire Hazard]
Flash point data for this chemical are not available; however, NICOTINIC ACID is probably combustible. | [Description]
Niacin is an additive to food on the basis of its nutrient supplement qualities as a vitamin (as an enzyme co-factor). This water-soluble vitamin of the B complex occurs in various animal and plant tissues. It is required by the body for the formation of coenzymes NAD and NADP. A deficiency of niacin results in the disease, pellagra. | [Physical properties]
Nicotinic acid and nicotinamide are colorless crystalline substances. Each is insol uble or only sparingly soluble in organic solvents. Nicotinic acid is slightly soluble
in water and ethanol; nicotinamide is very soluble in water and moderately soluble
in ethanol
Nicotinic acid is amphoteric and forms salts with acids as well as bases. Its car boxyl group can form esters and anhydrides and can be reduced. Both nicotinic acid
and nicotinamide are very stable in dry form, but in solution nicotinamide is hydro lyzed by acids and bases to yield nicotinic ac
The coenzyme forms of niacin are the pyridine nucleotides, NAD(H) and
NADP(H). In each of these compounds, the electron-withdrawing effect of the N-1
atom and the amide group of the oxidized pyridine nucleus enables the pyridine C-4
atom to react with many nucleophilic agents (e.g., sulfite, cyanide, and hydride
ions). It is the reaction with hydride ions (H?) that is the basis of the enzymatic
hydrogen transfer by the pyridine nucleotides; the reaction involves the transfer of
two electrons in a single step
Several substituted pyridines are antagonists of niacin in biological systems:
pyridine-3-sulfonic acid, 3-acetylpyridine, isonicotinic acid hydrazine, 17 and
6-aminonicotinamide | [History]
Huber first synthesized nicotinic acid in 1867. In 1914, Funk isolated nicotinic acid from rice polishings. Goldberger, in 1915, demonstrated that pellagra is a nutritional deficiency. In 1917, Chittenden and Underhill demonstrated that canine blacktongue is similar to pellagra. In 1935, Warburg and Christian showed that niacinamide is essential in hydrogen transport as diphosphopyridine nucleotide (DPN). In the following year, Euler et al. isolated DPN and determined its structure. In 1937, Elvhehjem et al. cured blacktongue by administration of niacinamide derived from liver. In the same year, Fouts et al. cured pellagra with niacinamide. In 1947, Handley and Bond established conversion of tryptophan to niacin by animal tissues. | [Application]
Nicotinic acid is a precursor of the coenzymes NAD and NADP. Widely distributed in nature; appreciable amounts are found in liver , fish, yeast and cereal grains. It is a water-soluble b-complex vitamin that is necessary for the growth and health of tissues. Dietary deficiency is associated with pellagra. It was functions as a nutrient and dietary supplement that prevents pellagra. The term "niacin" has also been applied. The term “niacin” has also been applied to nicotinamide or to other derivatives exhibiting the biological activity of nicotinic acid. | [Definition]
ChEBI: A pyridinemonocarboxylic acid that is pyridine in which the hydrogen at position 3 is replaced by a carboxy group. | [Brand name]
Niacor (Upsher Smith); Niaspan
(KOS); Nicolar (Sanofi Aventis); Wampocap (Medpointe). | [Biological Activity]
Nicotinic acid can be converted to nicotinamide in the animal body and, in this form, is found as a component of two oxidation-reduction coenzymes, NAD and NADP.The nicotinamide portion of the coenzyme transfers hydrogens by alternating between an oxidized quaternary nitrogen and a reduced tertiary nitrogen. Enzymes that contain NAD or NADP are usually called dehydrogenases. They participate in many biochemical reactions of lipid, carbohydrate, and protein metabolism. An example of an NAD-requiring system is lactic dehydrogenase which catalyzes the conversion of lactic acid to pyruvic acid. | [Biochem/physiol Actions]
Nicotinic is an antioxidant and acts as a coenzyme in the form of nicotinamide adenine nucleotides(NAD). It modulates lipid metabolism and may be useful in treating dyslipidemia. Nicotinic acid reduces the low-density lipoprotein (LDL) synthesis and improves high-density lipoprotein (HDL) levels. Deficiency of niacin leads to enhanced lipid peroxidation and is implicated in Crohn′s disease Deficiency also impacts DNA repair and also leads to skin and gastrointestinal disorder pellagra. | [Mechanism of action]
Nicotinic acid decreases formation and secretion of
VLDL by the liver.This action
appears secondary to its ability to inhibit fatty acid
mobilization from adipose tissue. Circulating free fatty
acids provide the main source of fatty acids for hepatic triglyceride synthesis, and lowering triglyceride synthesis
lowers VLDL formation and secretion by the liver.
Since plasma VLDL is the source of LDL, lowering
VLDL can ultimately lower LDL. In addition, nicotinic
acid shifts LDL particles to larger (more buoyant) sizes.
The larger LDL particles are thought to be less atherogenic.
Nicotinic acid can also significantly increase
plasma HDL levels; the mechanism is unknown. | [Pharmacokinetics]
Nicotinic acid is readily absorbed. Peripheral vasodilation is seen within 20 minutes, and peak plasma concentrations occur within 45
minutes. The half-life of the compound is approximately one hour, thus necessitating frequent dosing or an extended-release
formulation. Extended release tablets produce peripheral vasodilation within 1 hour, reach peak plasma concentrations within 4 to 5
hours, and have a duration of 8 to 10 hours.
Dosing of nicotinic acid should be titrated to minimize adverse effects. An initial dose of 50 to 100 mg t.i.d. often is used with immediate�release tablets. The dose then is gradually increased by 50 to 100 mg every 3 to 14 days, up to a maximum of 6 g/day, as tolerated.
Therapeutic monitoring to assess efficacy and prevent toxicity is essential until a stable and effective dose is reached. Similar dosing
escalations are available for extended-release products, with doses normally starting at 500 mg once daily at bedtime.. | [Clinical Use]
Nicotinic acid has been esterified to prolong itshypolipidemic effect. Pentaerythritol tetranicotinate hasbeen more effective experimentally than niacin in reducingcholesterol levels in rabbits. Sorbitol and myo-inositolhexanicotinate polyesters have been used in the treatment ofpatients with atherosclerosis obliterans.The usual maintenance dose of niacin is 3 to 6 g/daygiven in three divided doses. The drug is usually given atmealtimes to reduce the gastric irritation that often accompanieslarge doses. | [Side effects]
Compliance with nicotinic acid therapy can be poor
because the drug can produce an intense cutaneous
flush. This can be reduced by beginning the drug in
stepped doses of 250 mg twice daily and increasing the
dose monthly by 500 to 1000 mg per day to a maximum
of 3000 mg per day.Taking nicotinic acid on a full stomach
(end of meal) and taking aspirin before dosage can
reduce the severity of flushing. Time-release forms of
nicotinic acid may also decrease cutaneous flushing.
Nicotinic acid can cause gastrointestinal (GI) distress,liver dysfunction (especially at high doses), decreased
glucose tolerance, hyperglycemia, and hyperuricemia.
Thus, it is contraindicated in patients with hepatic dysfunction,
peptic ulcer, hyperuricemia, or diabetes mellitus.
A paradox associated with nicotinic acid is that it is
the most widely available hypolipidemic drug (it is sold
over the counter), yet its use requires the closest management
by the physician. | [Synthesis]
Nicotinic acid, pyridine-3-carboxylic acid (20.2.9) is synthesized industrially
by heating a paraldehyde trimer of acetaldehyde, under pressure with ammonia,
which leads to the formation of 2-methyl-5-ethylpyridine, followed by oxidation with
nitric acid which gives the desired product.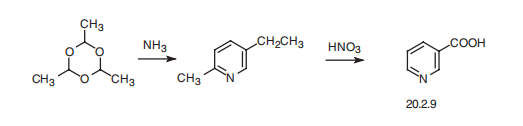
| [Metabolism]
Nicotinic acid is a B-complex vitamin that is converted to nicotinamide, NAD+
, and NADP+
.The latter two compounds are coenzymes and
are required for oxidation/reduction reactions in a variety of biochemical pathways. Additionally, nicotinic acid is metabolized to a
number of inactive compounds, including nicotinuric acid and N-methylated derivatives. Normal biochemical regulation and feedback
prevent large doses of nicotinic acid from producing excess quantities of NAD+
and NADP+
.Thus, small doses of nicotinic acid, such as
those used for dietary supplementation, will be primarily excreted as metabolites, whereas large doses, such as those used for the
treatment of hyperlipoproteinemia, will be primarily excreted unchanged by the kidney. | [storage]
Store at -20°C | [Purification Methods]
Crystallise the acid from *benzene, EtOH or H2O. It sublimes without decomposition. [McElvain Org Synth Coll Vol I 385 1941, Beilstein 22 III/IV 439, 22/2 V 57.] |
| Questions And Answer | Back Directory | [Chemical Properties]
Nicotinic acid, also known as niacin or vitamin B3, is a white crystal or crystalline powder, odorless or has a slight odor, slight sour taste. Melting point is 234-237℃. Easily soluble in hot water, hot ethanol, alkaline water, propylene glycol, and chloroform. Slightly soluble in water and ethanol; 100ml room temperature water can dissolve 1.6g. Insoluble in ether and ester solutions. The PH of 1% aqueous solution is 3.0-4.0. Stable in heat, acidity and alkalinity.
Nicotinic acid can produce a variety of adverse effects, depending on the intake and health of the consumer. The skin flushing reaction produced by nicotinic acid has been recognized for more than 70 years (Bean 1978). When taken on an empty stomach, crystalline nicotinic acid in doses as small as 10 mg may produce a mild and transient, but noticeable, flushing reaction. While not desirable, such reactions produce no known adverse consequences, and they are almost never perceptible when small amounts of nicotinic acid are taken in tablet or capsule form or consumed as part of food. | [Uses]
Nicotinic acid is an important factor in delivering hydrogen and fighting pellagra in organisms; it helps maintain skin and nerve health and stimulate digestion.
Nicotinic acid or niacinamide are used to treat and prevent pellagra. This is a disease caused by niacin deficiency. Niacin is also used to treat high cholesterol. In some cases, niacin taken with colestipol can work as well as colestipol and a statin medicine.
Niacin USP granular is used for food fortification, as dietary supplement and as an intermediate of pharmaceuticals.
Niacin feed grade is used as vitamin for poultry, swines, ruminants, fish, dogs and cats, etc. It is also used as intermediate for nicotinic acid derivatives and technical applications. | [Preparation]
Nicotinic acid exists naturally in grain germs, meats and peanuts. It can also be synthesized artificially through the liquid phase method (potassium permanganate oxidation and nitric acid oxidation) and gas phase method (ozone oxidation, ammonia oxidation and air oxidation).
In the gas phase ammonia oxidation process, add 3-methyl pyridine, air and ammonia into the fluidized bed reactor and catalyze the reaction at 290~360℃,V2O5 to produce nicotinonitrile; then hydrolyze in sodium hydroxide aqueous solution at 160℃ to produce sodium nicotinate; finally, add hydrochloric acid to acidify, creating nicotinic acid. In the potassium permanganate oxidation method, add potassium permanganate gradually at 80℃ to a mixture of 3-methyl pyridine and water, and then continue to mix for 30min at 85~90℃. Distill to collect and reuse the unreacted 3-methyl pyridine and filter away the produced manganese dioxide. Adjust the PH of the resulting nicotinic acid solution to 3.8~4.0 using hydrochloric acid, cool to 30℃ crystals, and filter to obtain crude nicotinic acid. Dissolve the crude nicotinic acid in hot water, add activated charcoal to eliminate the color, filter, cool, and obtain the crystalline end product. Yield is approximately 86%.
- 6- hydroxyquinoline method
Add sulfuric acid and quinoline into a reaction kettle and mix while maintaining heat at 150~160℃ for 5h. Then with the temperature maintained at 180~220℃, slowly drop in nitric acid and the sulfuric acid mixture over the course of 36~40h. While maintaining the temperature, mix for 2~3h to obtain a nicotinic acid solution and add water to dilute the solution. Use 30%~33% NaOH solution to neutralize the PH to 8~9. Cool and filter away the sodium sulfate and sodium nitrate crystals, add copper sulfate solution to the filtered liquid, and mix and heat to yield copper nicotinate precipitation. Cool, filter and add the copper nicotinate to an adequate amount of water, drop in NaOH solution until PH>9 and the liquid is no longer blue, and filter away the produced cupric oxide. Add a small amount of sodium sulfide solution to remove traces of copper and iron until the solution no longer produces black precipitate, and then filter. Use hydrochloric acid to adjust the PH of the filtered liquid to 3.5~3.9, filter to yield crystals as crude nicotinic acid. Dissolve the crude product in 12 times the amount of distilled water, add activated charcoal to eliminate the color, filter, cool, and obtain the crystalline end product. Yield is 35%~39%.
- 2-methyl-5-ethyl pyridine method
With 2-methyl-5-ethyl pyridine as the raw ingredient, oxidize with nitic acid under high pressure and high temperatures, then decarboxylate to yield nicotinic acid.
| [Identifying tests]
Add 2 portions of 2, 4-Dinitrochlorobenzene to the sample and process into powder. Place 10mg of the powder in a test tube, gently heat until melted, and continue to heat for a couple of seconds. Cool and add 3ml potassium hydroxide ethanol solution (TS-190). The solution should be dark red.
Dissolve 50mg of the sample solution in 20ml water, use 0.1mol/L sodium hydroxide to neutralize until a litmus paper reads neutral, and add 3ml copper sulfate solution (TS-78). Blue precipitate should begin forming slowly.
Dry the sample for 1h at 105℃ and collect its mineral oil dispersions. The peak wavelength of its infrared absorption spectrum should resemble the standard reference sample formulated using the same method.
Prepare an aqueous solution of the sample with a density of 20μg/ml, measure its absorbance at the wavelengths 237nm and 262nm in a 1cm pool, using water as a blank control. A237/A262 should be 0.35~0.39.
| [Content analysis]
Precisely take a sample of 300mg and dissolve in 50ml water. Add a couple drops of phenolphthalein solution (TS-167) and titrate using 0.1mol/L sodium hydroxide. Conduct a control experiment at the same time. Every Ml0.1mol/L sodium hydroxide is equivalent to 12.31mg nicotinic acid (C6H5NO2).
| [Toxicity]
LD50 7.0g/kg (Large mice, oral).
GRAS(FDA��,§182.5530����,2000)�����。
ADI has no special regulations (EEC, 1990).
|
|
|