| Identification | More | [Name]
Lamivudine | [CAS]
134678-17-4 | [Synonyms]
2'-deoxy-3'-thiacytidine
(2r-cis)-4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1h)-pyrimidinone
3TC
3'-THIA-2',3'-DIDEOXYCYTIDINE
4-AMINO-1-((2R,5S)-2-HYDROXYMETHYL-[1,3]OXATHIOLAN-5-YL)-1H-PYRIMIDIN-2-ONE
(-)-BCH-189
(-)-BETA-L-2',3'-DIDEOXY-3'-THIACYTIDINE
(-)-B-L-2',3'-DIDEOXY-3'-THIACYTIDINE
(-)-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cystosine
(-)-2¢
(-)NGPB-21
(2R-cis)-4-amino-1-
,3¢
2(1H)-Pyrimidinone,4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-,(2R-cis)-
3¢
3'-thia-2',3'-dideoxcytidine
-deoxy-3¢
Epivir
GR109714X
-thia-2¢ | [EINECS(EC#)]
603-844-3 | [Molecular Formula]
C8H11N3O3S | [MDL Number]
MFCD00869739 | [Molecular Weight]
229.26 | [MOL File]
134678-17-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
177 °C | [alpha ]
D21 -135° (c = 0.38 in methanol) | [Boiling point ]
475.4±55.0 °C(Predicted) | [density ]
1.73±0.1 g/cm3(Predicted) | [refractive index ]
-142 ° (C=1, MeOH) | [Fp ]
9℃ | [storage temp. ]
Freezer | [solubility ]
water: soluble10mg/mL, clear | [form ]
powder | [pka]
13.83±0.10(Predicted) | [color ]
white to beige | [Water Solubility ]
70g/L(temperature not stated) | [Usage]
A reverse transcriptase inhibitor | [Merck ]
5352 | [BCS Class]
1,3 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. | [InChIKey]
JTEGQNOMFQHVDC-NKWVEPMBSA-N | [CAS DataBase Reference]
134678-17-4(CAS DataBase Reference) | [EPA Substance Registry System]
2(1H)-Pyrimidinone, 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]- (134678-17-4) |
| Hazard Information | Back Directory | [Description]
Lamivudine is a new generation orally active nucleoside analog launched in the U.S.A. for use in combination with zidovudine (AZT) as a first-line therapy for
patients with HIV infection. Lamivudine is rapidly converted to phosphorylated
metabolites in the body which act as inhibitors and chain terminators of HIV reverse
transcriptase (RT), the enzyme required for the replication of the HIV genome.
Lamivudine has similar inhibitory potency to RT as AZT but is 10 times less toxic and is
active against AZT-resistant strains of HIV. Combination therapy of lamivudine and AZT
produced a large decrease in blood-borne virus with an increase in CD4 cells, an effect
that can be sustained for 2 years. Since hepatitis B virus (HBV) also encodes a
polymerase with a RT function necessary for the conversion of a RNA replicative
intermediate to DNA, clinical efficacy has been reported for lamivudine in treating
patients with HBV infection. It was reported that the enantiomer of lamivudine is
equipotent against HIV but with considerably higher cytotoxicity. | [Chemical Properties]
White Crystalline Powder | [Originator]
BioChem Pharma (Canada) | [History]
Lamivudine[134678-17-4] is produced by GlaxoSmithKline LLC. In the early 1990s, it was used by some countries in Europe and North America to treat AIDS. In the mid-1990s, medical experts found that it had an inhibitory effect on the DNA of hepatitis B virus. In 1998, the US Food and Drug Administration (FDA) first approved it as a treatment drug for hepatitis B. In 1999, the China Food and Drug Administration approved this drug as a hepatitis B treatment drug, with the Chinese trade name "Heputin". | [Uses]
Lamivudine is used along with other medications to treat human immunodeficiency virus (HIV) infection in adults and children 3 months of age and older.
Lamivudine (Epivir-HBV) is used to treat hepatitis B infection.
Lamivudine is in a class of medications called nucleoside reverse transcriptase inhibitors (NRTIs). It works by decreasing the amount of HIV and hepatitis B in the blood. | [Definition]
ChEBI: A monothioacetal that consists of cytosine having a (2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl moiety attached at position 1. An inhibitor of HIV-1 reverse transcriptase. | [Indications]
Lamivudine is a synthetic cytidine analogue used in the
treatment of HIV and HBV. Its activation
requires phosphorylation by cellular enzymes.
Lamivudine triphosphate competitively inhibits HBV
DNA polymerase and HIV reverse transcriptase and
causes chain termination. It inhibits the activity of
mammalian DNA polymerases with a much lower potency.
HIV-1 frequently acquires mutations in reverse
transcriptase that result in resistance to lamivudine
within 12 weeks of treatment. Mutations in the DNA
polymerase of HBV are associated with decreased
lamivudine efficacy and have been documented in patients
treated with this agent for 6 months or more. | [Synthesis]
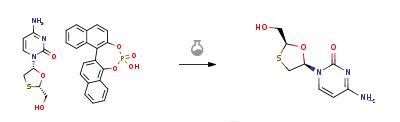
Aqueous hydrochloric acid (6N, 30 ml) was slowly added to a solution of 20 gm of the solid (2R-cis)-4-Amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidi- none.S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate in water (200 ml) at 45-50 deg C. Stirred the reaction for 1 hour at room temperature. The solid S-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate was filtered and the aqueous layer was neutralized with aqueous sodium hydroxide solution (30%, 20 ml). The solvent was recovered under vacuum at 40-45 deg C., the product obtained was dissolved in methanol (200 ml), filtered to remove the inorganic salts, the filtrate was concentrated under vacuum at 40-45 deg C. and the residual solid obtained was dissolved in ethanol (50 ml), heated to 50 deg C., slowly allowed to room temperature, cooled to 10 deg C., filtered and dried at 40-45 deg C. to obtain 5 gm of Lamivudine(Chiral purity: 97.5%).
 | [Manufacturing Process]
To a solution of potassium t-butoxide (0.11 mol) in 100 ml DMF was added
thiobenzoic acid (0.11 mol) and the solution partially evaporated in vacuo,
benzene added in two consecutive portions and evaporated in vacuo each
time. To the residual DMF solution was added bromoacetaldehyde diethyl
acetal (0.1 mol) and the mixture stirred at 120°C for 15 h. After cooling, it
was poured onto water (500 ml), the product extracted with ether, the extract
washed with aqueous NaHCO3 followed by water, then dried and the solvent
removed in vacuo. The residue was distilled in vacuo to give 17.2 g of pure 2-
thiobenzoyl acetaldehyde diethyl acetal, boiling point 131-133°C/0.07 mm.
The 2-thiobenzoyl acetaldehyde diethyl acetal (17.2 g) was dissolved in 100
ml THF followed by the addition of 6 g NaOH in 20 ml H2O. The mixture was
refluxed under N2 for 15 h, then cooled and diluted with water (200 ml) and
the product extracted with ether (3 x 200 ml). The extract was dried, the
solvent removed in vacuo and the residue distilled to yield 7.1 g of
mercaptoacetaldehyde diethylacetal.
50 g of the 1-benzoyl glycerol in a mixture of 500 ml of CH2Cl2 and 25 ml of
H2O was treated portionwise with 80 g of NaIO4 under vigorous stirring at
room temperature. After addition, stirring was continued for 2 h after which
time 100 g of MgSO4 was added and stirring continued for 30 min. The
mixture was filtered, the filtrate evaporated in vacuo and the residue distilled
to yield 26 g of pure benzoyloxyacetaldehyde, boiling point 92-94°C/0.25 mm.
2-Benzoyloxymethyl-5-ethoxy-1,3-oxathiolane:
The mercaptoacetaldehyde diethylacetal (7 g) was mixed in 100 ml of toluene
with 7 g of the above benzoyloxyacetaldehyde, a few crystals of p�toluenesulfonic acid added and the mixture place in an oil-bath at 120°C
under N2. The formed ethanol was allowed to distill over, the mixture kept at
120°C for 30 min longer than cooled and washed with aqueous NaHCO3, dried
and evaporated in vacuo. The residue was distilled in vacuo to yield 9.8 g of
2-benzoyloxymethyl-5-ethoxy-1,3-oxathiolane as a mixture of cis- and trans�isomers, boiling point 140-143°C/0.1 mm.
Cis- and trans-2-benzoyloxymethyl-5-cytosin-1'-yl-1,3-oxathiolane:
A mixture of 2.7 g of cytosine, 30 ml of hexamethyldisilazane (HMDS) and 0.3
ml of trimethylsilyl chloride (TMSCl) was heated under reflux under dry N2
untila clear solution resulted (3 L) and the excess reagents evaporated in
vacuo. The remaining volatiles were removed under high vacuum, the solid
residue taken up in 250 ml of dichlorethane and 5 g of the 2-benzoyloxymethyl-5-ethoxy-1,3-oxathiolane in 50 ml of dichloroethane added
under dry argon followed by 4.7 ml of trimethylsilyl triflate. After 3 days of
heating under reflux under argon, it was cooled and poured onto 300 ml of
saturated aqueous NaHCO3. The organic layer was collected, the aqueous
phase extracted with CH2Cl2and the combined extracts washed with water,
dried and evaporated in vacuo. The residue was purified by chromatography
on silica gel using CH2Cl2-CH3OH 9:1 as the eluant to give 2.5 g of a pure
mixture of cis- and trans-2-benzoyloxymethyl-5-cytosin-1'-yl-1,3-oxathiolane
in a 1:1 ratio. These were separated as the N-acetyl derivatives.
The preceding mixture of cis- and trans-2-benzoyloxymethyl-5-cytosin-1'-yl-
1,3-oxathiolane (2.5 g) in 100 ml of dry pyridine containing 0.1 g of 4-
dimethylaminopyridine (DMAP) was treated with acetic anhydride (7 ml) at
room temperature and after 16 h, the mixture was poured onto cold water
followed by extraction with CH2Cl2. The extract was washed with water, dried,
and evaporated in vacuo. Toluene was added to the residue, then evaporated
in vacuo and the residual oil purified by chromatography on silica gel using
EtOAc-CH3OH 99:1 as the eluant to yield 1.35 g of pure trans-2-
benzoyloxymethyl-5-(N4-acetyl-cytosin-1'-yl)-1,3-oxathiolaneas the fast
moving product and 1.20 g of pure cis-2-benzoyloxymethyl-5-cytosin-1'-yl-
1,3-oxathiolan as the slow moving component, melting point 158-160°C.
Cis- and trans-isomers of 2-hydroxymethyl-5-(cytosin-1'-yl)-1,3-oxathiolane
was obtained by action of methanolic ammonia at 24°C. | [Brand name]
Epivir (GlaxoSmithKline). | [Therapeutic Function]
Antiviral | [Acquired resistance]
A single codon change at position 184 in the HIV reverse
transcriptase gene confers high-level resistance. The K65R
mutation is also associated with resistance. In-vitro data indicate
that lamivudine resistance may restore HIV sensitivity to
zidovudine- and tenofovir-resistant virus. | [General Description]
Lamivudine is (-)-2',3'-dideoxy-3'-thiacytidine, (-)-β-L-(2R,5S)-1,3-oxathiolanylcytosine, 3TC, or (-)-(S)-ddC.Lamivudine is a synthetic nucleoside analog that differsfrom 2β,3β-dideoxycytidine (ddC) by the substitution of asulfur atom in place of a methylene group at the 3'-positionof the ribose ring. In early clinical trials, lamivudine exhibitedhighly promising antiretroviral activity against HIVand low toxicity in the dosages studied.Preliminarypharmacokinetic studies indicated that it exhibited goodoral bioavailability (F=~80%) and a plasma half-life of 2to 4 hours. | [Pharmaceutical Applications]
An analog of cytidine available for oral administration. | [Biochem/physiol Actions]
Lamivudine is a potent nucleoside analog reverse transcriptase inhibitor (nRTI). It is an analogue of cytidine, and can inhibit both types (1 and 2) of HIV reverse transcriptase as well as the reverse transcriptase of hepatitis B. It needs to be phosphorylated to its triphosphate form before it is active. 3TC-triphosphate also inhibits cellular DNA polymerase. | [Pharmacokinetics]
The pharmacokinetics of lamivudine are similar in patients with HIV-1 or HBV infection, and healthy volunteers. The drug is rapidly absorbed after oral administration, with maximum serum concentrations usually attained 0.5 to 1.5 hours after the dose. | [Clinical Use]
Lamivudine is indicated for the treatment of HIV when
used in combination with other antiretroviral agents.A
lower dose than that used to treat HIV is approved for
the treatment of HBV. Although lamivudine initially
improves histological and biochemical measures of hepatic
function and reduces HBV DNA to below the limits
of detection, withdrawal of the drug usually results in
disease recurrence. Resistance appears in up to onethird
of patients after 1 year of treatment. | [Side effects]
Lamivudine is relatively safe and non-toxic. Animal studies of very high doses did not result in any organ toxicity. In patients co-infected with HIV and HBV, cessation of lamivudine therapy may result in clinical and/or laboratory evidence of recurrent hepatic disease that may be more severe in patients with hepatic decompensation. Tests of liver function and inflammation and markers of HBV replication should be periodically monitored.
Lamivudine competes with emtricitabine for the enzymes involved in intracellular phosphorylation and co-administration is contraindicated. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: trimethoprim inhibits excretion of
lamivudine - avoid concomitant use of high dose
co-trimoxazole.
Antivirals: avoid concomitant use with foscarnet,
emtricitabine and IV ganciclovir.
Cytotoxics: avoid with cladribine.
Orlistat: absorption possibly reduced by orlistat. | [Metabolism]
Lamivudine is metabolised intracellularly to the active
antiviral triphosphate. Hepatic metabolism is low
(5-10%) and the majority of lamivudine is excreted
unchanged in the urine via glomerular filtration and active
secretion (organic cationic transport system). | [storage]
Store at -20°C | [References]
Arts and Wainberg (1996), Mechanism of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription; Antimicrob. Agents Chemother., 40 527
Coates et al. (1992), (-)-2’-deoxy-3’-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro; Antimicrob. Agents Chemother., 36 733
Kong et al. (2022), Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy; Cell Death Dis., 13 336
Rajukar et al. (2022), Reverse Transcriptase Inhibition Disrupts Repeat Element Life Cycle in Colorectal Cancer; Cancer Discov., 2021 Online ahead of print |
|
|