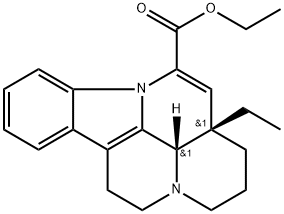
Vinpocetine synthesis
- Product Name:Vinpocetine
- CAS Number:42971-09-5
- Molecular formula:C22H26N2O2
- Molecular Weight:350.46
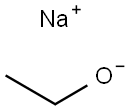
141-52-6
466 suppliers
$10.00/5g
1617-90-9
402 suppliers
$6.00/25mg

42971-09-5
484 suppliers
$5.00/100mg
Yield:42971-09-5 43.2 kg
Reaction Conditions:
with sulfuric acid;acetic acid in ethanol at 70; pH=2 - 14; for 3 h;Reflux;Large scale;
Steps:
1
in 500l of reaction kettle sequentially added 200 L of anhydrous ethanol, Reaction raw material vincamine 50 kg, Catalyst Sodium ethoxide 3.0 kg then keep the system pH at about 14 and slowly raised temperature to 70 ° C and allow to react for 3 h. Thin layer chromatography monitoring of raw materials vincamine fully reacted. As the hydrolysis reaction is completed, the product is vincamine acid and dehydrated vincamine mixture. Continue in the same reactor, stirring and cooling below 10 ° C, adjusting ph to about 12 by adding glacial acetic acid then again raise the temperature to reflux and allowed to react for 2h. TLC analysis of vincamine acid point of not more than 5%, as dehydration reaction is completed. Obtained a product of dehydrating vincamine acid. Continue in the same reactor, stirring down 10 ° C below then the addition of acetic anhydride 14.5 and the reaction of water was generated. Then added acetic acid to adjust pH , stirred the reaction for 1h and system pH value was about 7. Then add concentrated sulfuric acid to adjust the pH value to about 2, and then heated to 65-75 ° C and allowed to react for 6-8 h. TLC analysis of raw materials dehydrated vincamine acid intermediate reaction completed and completed as ethyl esterification reaction. The reaction system was stirred and cooled below 10 ° C, adjust the pH to about 7 by adding 30% of sodium hydroxide solution as vinpocetine synthesis steps to complete.. The synthesis step of vincristine ethanol solution into the concentrator, concentrated under reduced pressure recovery of ethanol. Then add 200L methylene chloride and 200L water, into the extraction tank for extraction and separation, dichloromethane extraction separation twice, the organic phase, dried with anhydrous sodium sulfate. And then transferred to the mixer with a mixer to recover dichloromethane to dry, get vinca cis-crude. Then add 450L of anhydrous ethanol to dissolve, fine filter, the filtrate into the crystallization tank with a concentrator. After evaporation of 300 L of ethanol under reduced pressure, the mixture was cooled to below 10 ° C for 2 h, filtered and dried to obtain 43.2 kg of vincristine and 86.4% by weight. HPLC detection purity of 99.3%, the external standard method measured content of 99.5%.
References:
CN103880838,2016,B Location in patent:Paragraph 0012; 0013

141-52-6
466 suppliers
$10.00/5g
28152-73-0
26 suppliers
inquiry

42971-09-5
484 suppliers
$5.00/100mg

141-52-6
466 suppliers
$10.00/5g
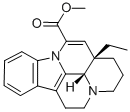
4880-92-6
54 suppliers
$105.00/10mg

42971-09-5
484 suppliers
$5.00/100mg

64-17-5
724 suppliers
$10.00/50g

4880-92-6
54 suppliers
$105.00/10mg

42971-09-5
484 suppliers
$5.00/100mg
![Indolo[2,3-a]quinolizine-1-propanoic acid, 1-ethyl-1,2,3,4,6,7,12,12b-octahydro-α-(hydroxyimino)-, ethyl ester, (αE,1S,12bS)-](/CAS/20210305/GIF/89396-73-6.gif)
89396-73-6
0 suppliers
inquiry

42971-09-5
484 suppliers
$5.00/100mg