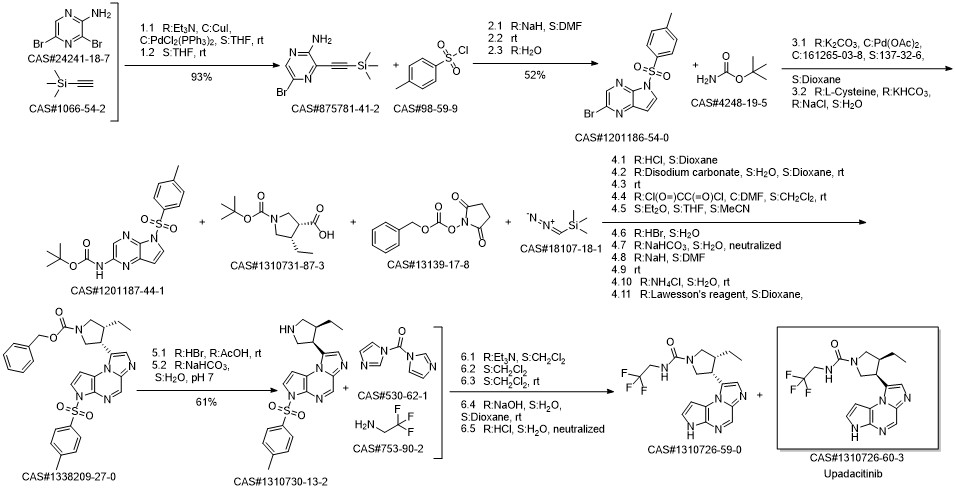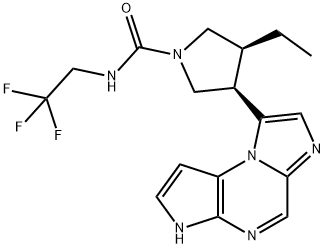
Upadacitinib synthesis
- Product Name:Upadacitinib
- CAS Number:1310726-60-3
- Molecular formula:C17H19F3N6O
- Molecular Weight:380.37
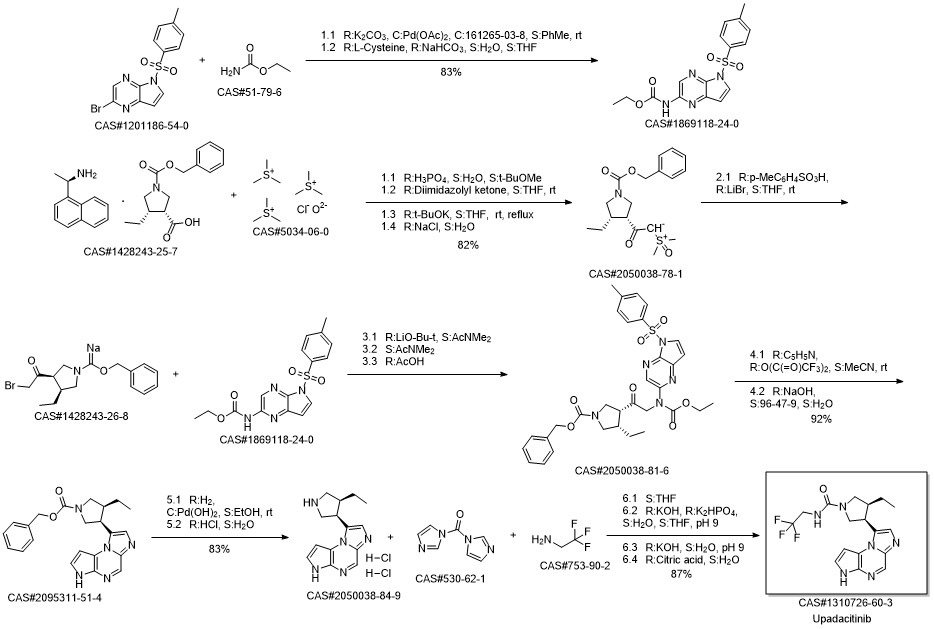
Reference: Pangan, Aileen L.; Teixeira, Henrique D.; Allian, Ayman D.; Borchardt, Thomas B.; Jayanth, Jayanthy; Marroum, Patrick J.; Nordstrom, Fredrik Lars; Sheikh, Ahmad Y.; Mohamed, Mohamed-Eslam F.; Othman, Ahmed A.; Mayer, Peter T. Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof for disease treatment. Assignee AbbVie Inc., USA. US 20180298016. (2018).
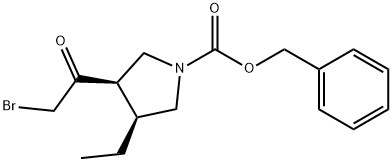
1428243-26-8
212 suppliers
inquiry

1310726-60-3
327 suppliers
$28.00/1mg
Yield:-
Steps:
Multi-step reaction with 4 steps
1.1: lithium tert-butoxide / N,N-dimethyl acetamide / 0.5 h / -10 - 5 °C
1.2: 1 h / -10 °C
2.1: pyridine; trifluoroacetic anhydride / acetonitrile / 2 h / 75 °C
3.1: 10% palladium hydroxide on charcoal; hydrogen / ethanol / 16 h / 50 °C / 1794.37 Torr
3.2: 1 h
4.1: tetrahydrofuran / 1.33 h / 30 °C
4.2: 1 h / 25 °C / pH 9
References:
US2017/129902,2017,A1

1201186-54-0
180 suppliers
$41.00/100mg

1310726-60-3
327 suppliers
$28.00/1mg
![tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate](/CAS/20200119/GIF/1201187-44-1.gif)
1201187-44-1
218 suppliers
$220.00/250mg

1310726-60-3
327 suppliers
$28.00/1mg
![Phenylmethyl (3R,4S)-3-[2-[[(1,1-dimethylethoxy)carbonyl][5-[(4-methylphenyl)sulfonyl]-5H-pyrrolo[2,3-b]pyrazin-2-yl]amino]acetyl]-4-ethyl-1-pyrrolidinecarboxylate](/CAS/20200611/GIF/1428243-27-9.gif)
1428243-27-9
39 suppliers
inquiry

1310726-60-3
327 suppliers
$28.00/1mg