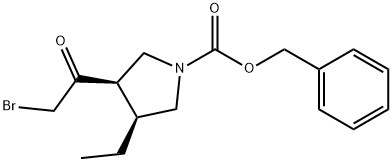
(3R,4S)-3-(2-Bromoacetyl)-4-ethyl-1-pyrrolidinecarboxylic acid phenylmethyl ester synthesis
- Product Name:(3R,4S)-3-(2-Bromoacetyl)-4-ethyl-1-pyrrolidinecarboxylic acid phenylmethyl ester
- CAS Number:1428243-26-8
- Molecular formula:C16H20BrNO3
- Molecular Weight:354.24
![(3R,4S)-1-[(benzyloxy)carbonyl]-4-ethylpyrrolidine-3-carboxylic acid](/CAS/20180531/GIF/1428243-25-7.gif)
1428243-25-7
30 suppliers
inquiry
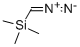
18107-18-1
212 suppliers
$33.00/5g

1428243-26-8
212 suppliers
inquiry
Yield:1428243-26-8 70 g
Reaction Conditions:
Stage #1: (3R,4S)-1-((benzyloxy)carbonyl)-4-ethylpyrrolidine-3-carboxylic acid (R)-1-(naphthalen-1-yl)ethan-1-amine saltwith hydrogenchloride in dichloromethane;water;
Stage #2: with oxalyl dichloride in dichloromethane at 50; for 0.166667 h;
Stage #3: diazomethyl-trimethyl-silaneFurther stages;
Steps:
18 Example- 18: Preparation of benzyl (3R,4S)-3- (2-bromoacetyl) -4-ethylpyrrolidine- 1- carboxylate
iN HC1 (1090 mL) & dichioromethane (500 mL) were added to (R)-1-(naphthalen-1-yl) ethan- 1-amine salt of (3R,4S)- 1 -((benzyloxy)carbonyl)-4-ethylpyrrolidine-3 -carboxylic acid (109 g) and stirred. The organic layer was separated and aqueous layer was extracted with dichloromethane (1090 mL). Combined organic layer was washed with iN HC1 (1090 mL), brine solution (1090 mL) and dried over sodium sulfate. The solvent was removed under reduced pressure at 40°C to obtain crude product. The crude product was dissolved in dichloromethane (654 mL) and cooled to 5°C. 2M oxalyl chloride in dichloromethane (243 mL) was added at 5°C and stirred for 10 mm at the same temperature. Dimethyl formamide (0.2 mL) was added at 5°C and stirred at 27°C for 6 hours. The solvent was removed under reduced pressure at 45°C and diluted the reaction mixture with tetrahydrofuran and acetonitrile and cooled to -10°C under nitrogen atmosphere. 2M Trimethylsilyldiazomethane in ether (486.6 mL) was added at -10°C and stirred for 3 hours at the same temperature. 47% aqueous HBr was added at -5°C and stirred for 3 hours. After the completion of reaction, water (1090 mL) and methyl tert.butyl ether (1090 mL) was added at 0°C and separated the organic layer. The organic layer was washed with saturated sodium bicarbonate solution, brine solution and dried over sodium sulfate. The solvent was removed from the organic layer under reduced pressure and purified the crude product by column chromatography using 15%ethyl acetate-hexane as eluent to obtain the title compound as orange solid. Yield: 70 g
References:
WO2019/16745,2019,A1 Location in patent:Page/Page column 35; 36
55314-57-3
107 suppliers
$23.00/1g

1428243-26-8
212 suppliers
inquiry
1145-81-9
152 suppliers
$139.00/5G

1428243-26-8
212 suppliers
inquiry