
N-Tosyl-5-bromo-4,7-diazaindole synthesis
- Product Name:N-Tosyl-5-bromo-4,7-diazaindole
- CAS Number:1201186-54-0
- Molecular formula:C13H10BrN3O2S
- Molecular Weight:352.21

875781-43-4
274 suppliers
$19.00/1g
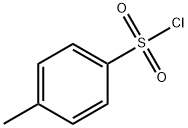
98-59-9
613 suppliers
$9.00/5g

1201186-54-0
177 suppliers
$41.00/100mg
Yield:1201186-54-0 97%
Reaction Conditions:
Stage #1:2-bromo-5H-pyrrolo[2,3-b]pyrazine with sodium hydride in N,N-dimethyl-formamide at 0 - 5; for 0.5 h;
Stage #2:p-toluenesulfonyl chloride in N,N-dimethyl-formamide at 0 - 20;
Steps:
7.A
A solution of 2-bromo-5H-pyrrolo[2,3-b]pyrazine (78.0 g, 394 mmol, Ark Pharm) in anhydrous DMF (272 mL) was added drop-wise over about 60 min to a stirred suspension of NaH (12.8 g, 532 mmol) in anhydrous DMF (543 mL) at about 0-5° C. The brown reaction solution was stirred for about 30 min at about 0-5° C. then a solution of p-toluenesulfonyl chloride (94.0 g, 492 mmol) in anhydrous DMF (272 mL) was added drop-wise over about 60 min at about 0-5° C. The reaction mixture was stirred at about 0-5° C. for about 1 h then allowed to warm to ambient temperature and stirred for about 18 h at ambient temperature. The reaction mixture was poured slowly into ice water (6 L), followed by the addition of aqueous 2.5 N NaOH (50.0 mL, 125 mmol). The precipitate was collected by filtration and stirred with cold water (3×200 mL). The solid was collected by filtration and dried to constant weight in a vacuum oven at about 55° C. to yield 2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine (134.6 g, 97%) as a pale beige solid: LC/MS (Table 2, Method d) Rt=1.58 min; MS m/z: 352/354 (M+H)+.
References:
ABBOTT LABORATORIES US2009/312338, 2009, A1 Location in patent:Page/Page column 60
875781-41-2
102 suppliers
$20.00/100mg

98-59-9
613 suppliers
$9.00/5g

1201186-54-0
177 suppliers
$41.00/100mg
![5-[(4-methylphenyl)sulfonyl]-5H-Pyrrolo[2,3-b]pyrazin-2-amine](/CAS/20180629/GIF/1201187-46-3.gif)
1201187-46-3
52 suppliers
inquiry

1201186-54-0
177 suppliers
$41.00/100mg