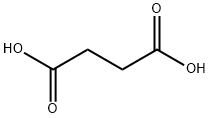
Succinic acid synthesis
- Product Name:Succinic acid
- CAS Number:110-15-6
- Molecular formula:C4H6O4
- Molecular Weight:118.09
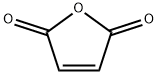
108-31-6
846 suppliers
$13.47/50g

110-15-6
961 suppliers
$5.00/10g
Yield:110-15-6 99.7%
Reaction Conditions:
with hydrogen;POUB-0.8 (0.8 wt percent palladium on carbon) modified with succinic acid in water at 120; under 10 - 15 Torr;Product distribution / selectivity;Industry scale;Inert atmosphere;Autoclave;
Steps:
5
EXAMPLE 5; 20 kg of maleic anhydride are dissolved in 40 liter of water in a suspensor.The reactor (V=100 dm3) is loaded with 20 liter of water acidified with succinic acid. The concentration of acid in water is 0.012 wt. %. The reactor is purged with nitrogen and then filled with a nitrogen-hydrogen mixture (1:1, volume), followed by loading of POUB-08 catalyst (300 g in recalculation to dry mass), whose active phase, according to the specification, is palladium in reduced form. The catalyst suspension in the diluted solution of succinic acid is preserved under continuous stiffing for 30-40 min, with temperature being gradually increased to 40-50° C.After modification of catalyst is finished, the reactor is fed a water solution of maleic anhydride (maleic acid), which is pushed into the reactor under nitrogen pressure from the suspensor (suspension reactor). The nitrogen-hydrogen mixture in the reactor is replaced with hydrogen, and hydrogenation is carried out under a pressure 10-15 atm in a polythermal regime (temperature gradually rises to 120° C. at the expense of heat released in the reaction).After hydrogen consumption is finished and the reaction mass has remained for an hour under the same conditions (stiffing; hydrogen pressure, 10-15 atm; temperature, 100-120° C.), hydrogen is pushed out of the reactor with nitrogen, and the catalysate is extruded through a Druck-filter into a heated (90-100° C.) crystallizing tank, which is then supplemented with 20 ml of the seed water solution (a solution of synthetic succinic acid with certified biological activity obtained as described in Example 4). The concentration of seed in the entire mass is 0.001%. The process of crystallization is carried out in a regime of variable-rate temperature decreasing. The rate of cooling is regulated in such a way that approximately a half of succinic acid would get sedimented after 13-17 min. Then the rate of cooling is lowered to a level that would provide sedimentation of 70% of total succinic acid within 35-40 min since the beginning of cooling. The cooling is proceeded until temperature reaches 8-10° C. (the entire crystallization process takes 1.5-2 h), and the sediment is separated from the source solution on a Nutsche-filter, the solution returning into the cycle. The source solution is used both to prepare the initial solution of succinic anhydride and to modify the “fresh” catalyst. The acid is dried in a rack-dryer at a temperature 90-95° C. The resulted succinic acid (yield, 80%) is of high quality: assay, 100%; melting point, 187-187.5° C.; no unsaturated acids. With the source solution recycled three times, the yield of succinic acid reaches 99.7% of theoretically expected.
References:
US2011/213183,2011,A1 Location in patent:Page/Page column 3-4
6915-18-0
1 suppliers
inquiry

110-15-6
961 suppliers
$5.00/10g

110-16-7
720 suppliers
$10.00/5g

110-15-6
961 suppliers
$5.00/10g
110-17-8
1047 suppliers
$9.00/5g

110-15-6
961 suppliers
$5.00/10g
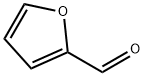
98-01-1
485 suppliers
$22.00/25g

110-15-6
961 suppliers
$5.00/10g