Maleic acid synthesis
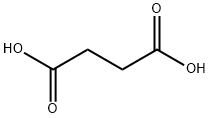
- Product Name:Maleic acid
- CAS Number:110-16-7
- Molecular formula:C4H4O4
- Molecular Weight:116.07
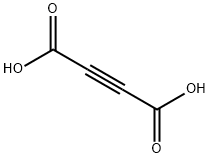
142-45-0
235 suppliers
$10.00/5g

110-16-7
720 suppliers
$10.00/5g
Yield:110-16-7 94%
Reaction Conditions:
with hydrogen in methanol at 20; under 760.051 Torr; for 5.5 h;Green chemistry;
Steps:
2.4. Partial hydrogenation of alkyne to corresponding alkene by functionalizedSBA-15/ metformin/ palladium catalyst
General procedure: 100 mg prepared catalyst mixed with 100 cc methanol was poured intothe flask and 20 mmol alkyne was added to this mixture. Hydrogenation set isfilled with H2 in atmospheric pressure and hydrogenating was continued for 5h. Progress of reaction was followed each 0.5 h by gas chromatography. Afterthe completion of reaction and disappearing of starting alkyne that took 4-6h, crude product was filtered. The filtrate was evaporated at low pressure byrotary evaporator. The products were characterized by 1H-NMR, 13C-NMR,FT-IR, MS, that strongly approved the (Z)-double bond configuration of producedalkene. The results of all experimental data associated with analysis; IR,1HNMR, 13C-NMR and Mass Spectrum are presented in the following:
References:
Kojoori, Reza Kia [Journal of the Chilean Chemical Society,2016,vol. 61,# 3,p. 3144 - 3149]
67-47-0
586 suppliers
$5.00/100mg

110-16-7
720 suppliers
$10.00/5g
98-01-1
485 suppliers
$22.00/25g

110-16-7
720 suppliers
$10.00/5g

98-01-1
485 suppliers
$22.00/25g
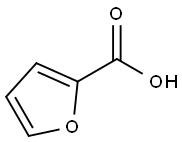
88-14-2
490 suppliers
$5.00/10g
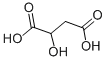
617-48-1
293 suppliers
$16.60/100G

110-16-7
720 suppliers
$10.00/5g