Peramivir synthesis
- Product Name:Peramivir
- CAS Number:229614-55-5
- Molecular formula:C15H28N4O4
- Molecular Weight:328.41
![Cyclopentanecarboxylic acid, 3-[(1S)-1-(acetylamino)-2-ethylbutyl]-4-[[bis[[(1,1-dimethylethoxy)carbonyl]amino]methylene]amino]-2-hydroxy-, (1S,2S,3R,4R)-](/CAS/20210305/GIF/383910-27-8.gif)
383910-27-8
0 suppliers
inquiry

229614-55-5
104 suppliers
inquiry
Yield:229614-55-5 73%
Reaction Conditions:
Stage #1: (-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylbutyl]-4-({[(tert-butoxycarbonyl)amino][(tert-butoxycarbonyl)-imino]methyl}amino)-2-hydroxycyclopentanecarboxylic Acidwith hydrogenchloride in water at 0;
Stage #2: with sodium hydroxide in water; pH=6 - 7;
Steps:
5
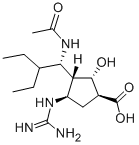
Example 55. (1^,2^,3^,4^)-3-(1-306αιηο-2-6Ηγ1 υγ1)-4^υαη(1ηο-2- hydroxycyclopent -anecarboxylic acid (Peramivir I)[0080] Compound 17 ( from example 4, 1.1 g, 2 mmol) was dissolved in aq. HC1 ( 6N, 6 mL, 36 mmol) at 0 °C. The mixture was stirred at room temperature overnight. The resulting solution was neutralized to pH 6-7 using ice-cold 1 N NaOH aq. solution. The mixture was concentrated to 1.5 ml by rotary evaporation. To this, methanol (20 mL) was added. The precipitate was filtered, and the filtrate was concentrated. The resulting white solid was recrystallized frommethanol/water (1 : 1, v/v) to give Peramivir I as a white solid.[0081] Yield: 500 mg (73%).[0082] MS (M+l ) : 329.[0083] H NMR (400 MHz, D20) δ 4.21 (d, J= 10.6 Hz, 2H), 3.70 (dd, J= 14.6, 9.0 Hz, 1H), 2.57 (d, J= 4.8 Hz, 1H), 2.40 (dt, J= 17.7, 8.9 Hz, 1H), 2.14 - 2.01 (m, 1H), 1.81 (s, 3H), 1.75 - 1.58 (m, 1H), 1.31 (s, 3H), 0.78 (ddd, J= 21.6, 18.6, 6.8 Hz, 8H). (See attached Chart 5)
References:
WO2012/145932,2012,A1 Location in patent:Page/Page column 28-29
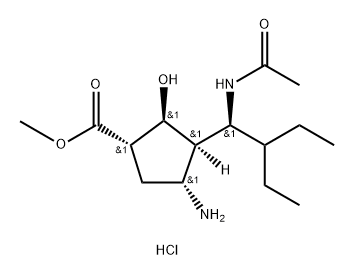
229614-17-9
8 suppliers
inquiry
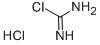
29671-92-9
133 suppliers
$5.00/250mg

229614-55-5
104 suppliers
inquiry
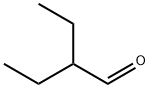
97-96-1
136 suppliers
$10.00/5mL

229614-55-5
104 suppliers
inquiry
![2-Cyclopentene-1-carboxylic acid, 4-[[bis[[(1,1-dimethylethoxy)carbonyl]amino]methylene]amino]-, methyl ester, (1S,4R)-](/CAS/20210305/GIF/1404223-97-7.gif)
1404223-97-7
0 suppliers
inquiry

229614-55-5
104 suppliers
inquiry
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
205 suppliers
$10.00/1g

229614-55-5
104 suppliers
inquiry