(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester synthesis
- Product Name:(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester
- CAS Number:229613-93-8
- Molecular formula:C18H30N2O5
- Molecular Weight:354.44
![(3aR,4R,6S,6aS)-4-(tert-butoxycarbonylaMino)-3-(pentan-3-yl)-4,5,6,6a-tetrahydro-3aH-cyclopenta[d]isoxazole-6-carboxylic acid](/CAS/GIF/316173-28-1.gif)
316173-28-1
59 suppliers
$355.00/100mg
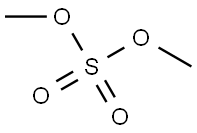
77-78-1
302 suppliers
$22.00/25g
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
205 suppliers
$10.00/1g
Yield:229613-93-8 97.13%
Reaction Conditions:
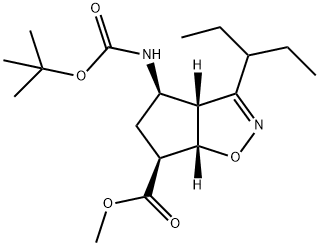
Stage #1: (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetrahydro-4H-cyclopent[d]isoxazole-6-carboxylic acid tert-butylaminewith potassium carbonate;sodium hydroxide in water;acetone;Industry scale;
Stage #2: dimethyl sulfate in water;acetone at 30 - 45;
Stage #3: with ammonia in methanol;water;acetone at 0 - 20; for 1.25 h;Industry scale;
Steps:
3 Synthesis of methyl (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetrahydro-4H-cyclopenta[d]isooxazole-6-carboxylate (5)
1,1-dimethylethyl ammonium (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy) carbonyl] amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetrahydro-4H-cyclopenta[d]isooxazole-6-carboxylate (16) (3210 g, 7.8 mol) was suspended in acetone (4000 g). Potassium carbonate (53.8 g), and an aqueous solution of 30% sodium hydroxide containing 312 g of sodium hydroxide were added to the resulting suspension. 3000 ml of solvent was removed by evaporation, 3000 ml of acetone was added, then, 3000 ml of solvent was removed by evaporation, 3000 ml of acetone was added, and thereafter 4000 ml acetone was removed by evaporation, obtaining a reaction solution substantially free of the odor of tert-butylamine. The reaction solution was cooled down to 30 - 35°C, to which 1060 ml of dimethyl sulfate was added dropwise at a speed to keep the temperature not exceeding 45°C. After completion of the addition, the resulting reaction mixture was stirred at 40 - 45°C for 1.5 hours. The reaction mixture was cooled down to 15 - 20°C, to which was then added 1500 ml of 25% ammonia. After stirring for 30 minutes, 1500 ml of methanol was added, and then the reaction mixture was cooled down to 0 - 5°C. Thereafter, 2240 ml of 25% ammonia was added to the reaction mixture within 45 minutes. The resulting product was collected by filtration, washed with 3000 ml of water, and dried under vacuum at 45 - 50°C. Finally, 2686.2 g of a product was obtained, with a yield of 97.13%.
References:
EP2186795,2010,A1 Location in patent:Page/Page column 6; 11-12
49805-57-4
0 suppliers
inquiry
![4-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylic acid methyl ester](/CAS/GIF/168683-02-1.gif)
168683-02-1
165 suppliers
$161.00/10g
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
205 suppliers
$10.00/1g
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
223 suppliers
$10.00/1g
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
205 suppliers
$10.00/1g
229613-83-6
42 suppliers
inquiry
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
205 suppliers
$10.00/1g
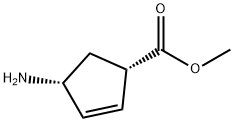
138923-03-2
33 suppliers
$333.00/250mg
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
205 suppliers
$10.00/1g