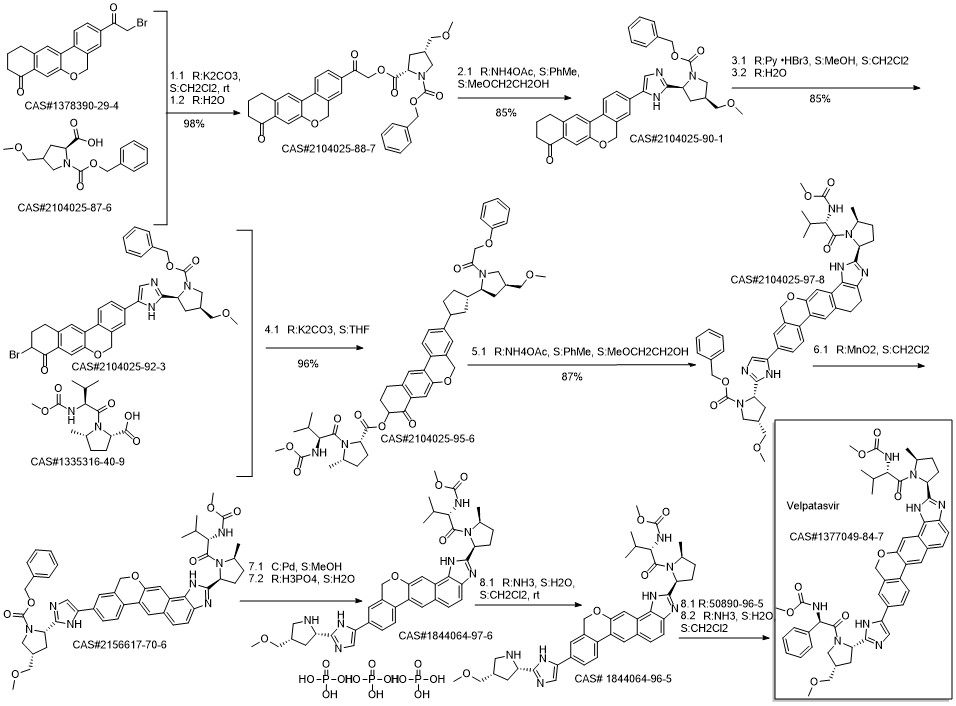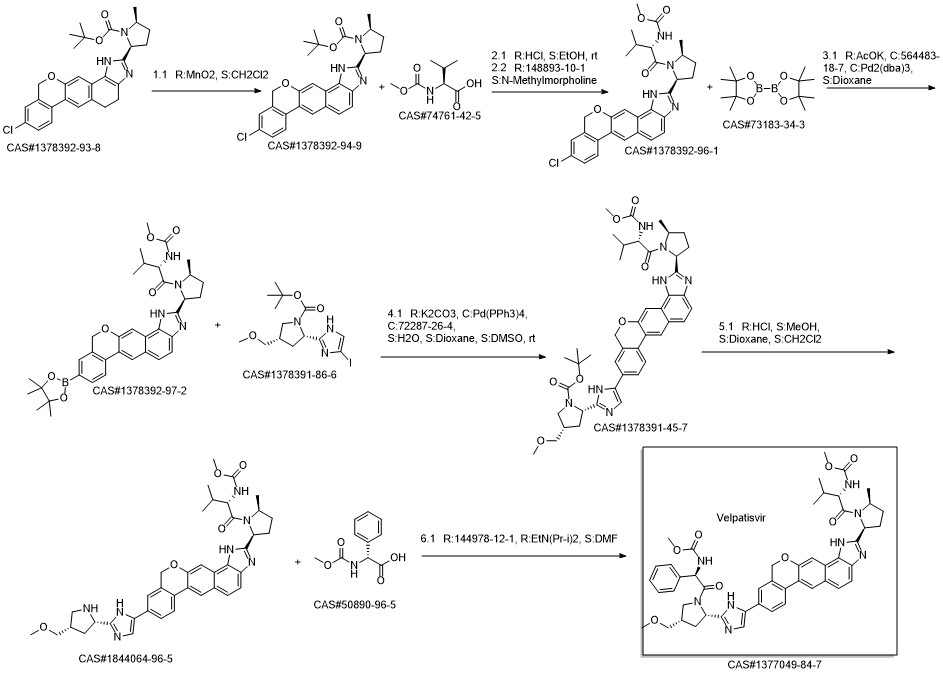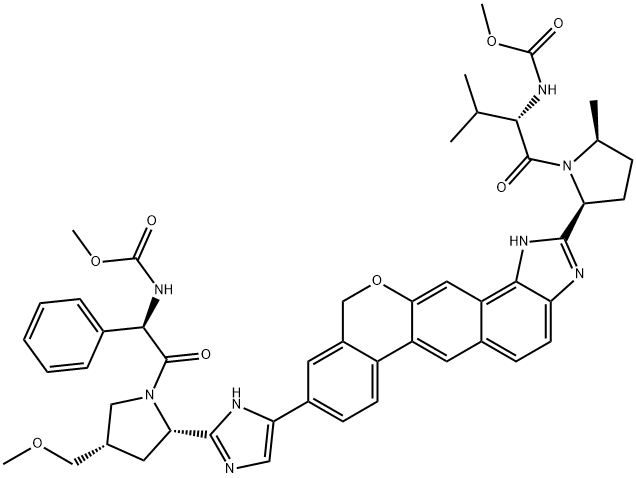
Velpatasvir synthesis
- Product Name:Velpatasvir
- CAS Number:1377049-84-7
- Molecular formula:C49H54N8O8
- Molecular Weight:883
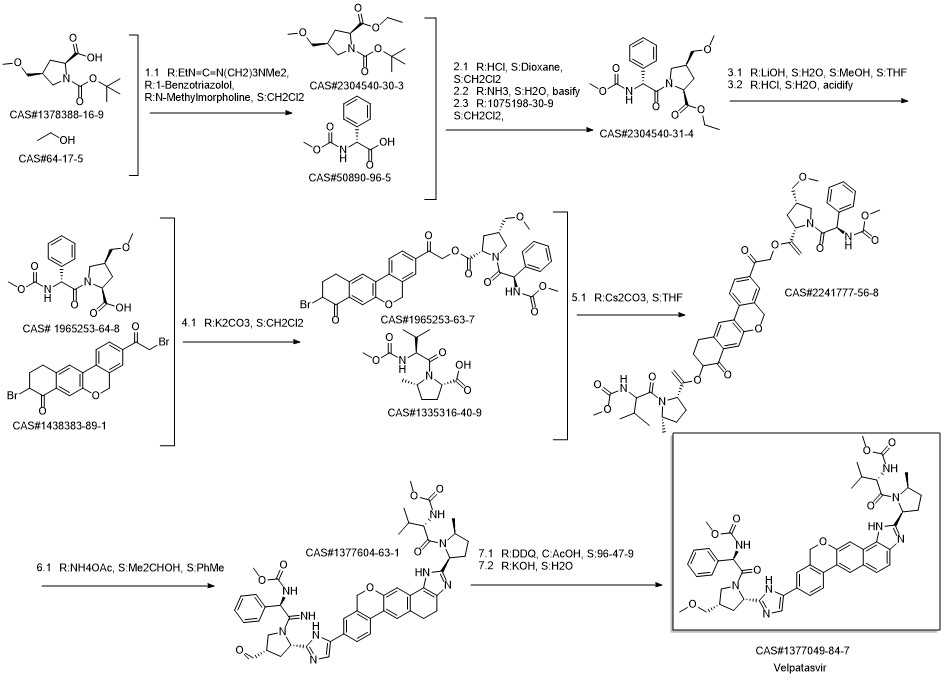
Reference: Reddy, Bandi Parthasaradhi; Reddy, Kura Rathnakar; Narasingam, Mogili; Krishna, Bandi Vamsi. Process for the preparation of velpatasvir. Assignee Hetero Research Foundation, India. IN 201641022433. (2018).
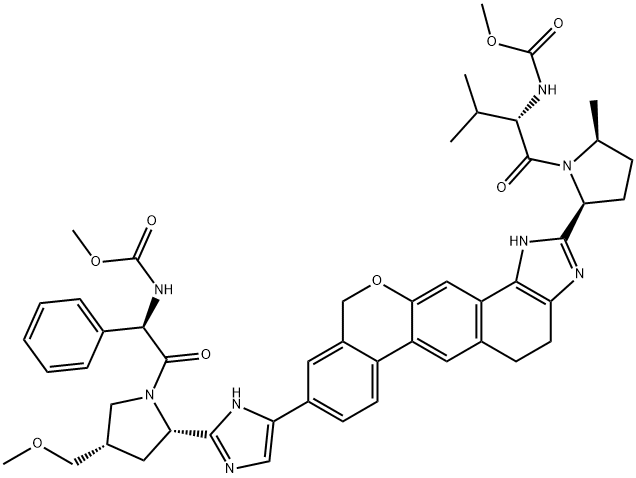
1377604-63-1
12 suppliers
inquiry

1377049-84-7
251 suppliers
$9.00/5mg
Yield:1377049-84-7 89%
Reaction Conditions:
with acetic acid;2,3-dicyano-5,6-dichloro-p-benzoquinone in tetrahydrofuran at 0 - 5;Inert atmosphere;
Steps:
1.5-3.5 Preparation of Velpatasvir:
Under the protection of nitrogen, add intermediate VP4 (23.3g, 26mmol, 1.0eq), 200mL of tetrahydrofuran to a 500mL reaction flask, stir to dissolve, cool to 0-5°C, add glacial acetic acid, and then add 50mL of DDQ tetrahydrofuran solution (8.9 g, 39 mmol, 1.5 eq). After the drop, the reaction was performed at 0-5°C, and the reaction was monitored by TLC. After the reaction was completed, 10% sodium hydroxide solution was added dropwise to adjust the pH to near neutral. 150 mL of water and 300 mL of dichloromethane were added, stirred, and allowed to stand for separation; the aqueous layer was extracted with dichloromethane (200 mL×3), and the organic phases were combined , Washed with saturated aqueous sodium chloride solution (200 mL×1), dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure to obtain a crude solid product. The crude product was recrystallized with 200 mL of isopropanol and water (2:1) to obtain 20.7 g of Velpatasvir solid product. Yield: 89%.
References:
Nantong Changyou Pharmaceutical Technology Co., Ltd.;Li Zebiao;Shen Minzhe;Ding Hongping;Pan Jing;Zou Lin CN110981879, 2020, A Location in patent:Page/Page column 6-8; 10
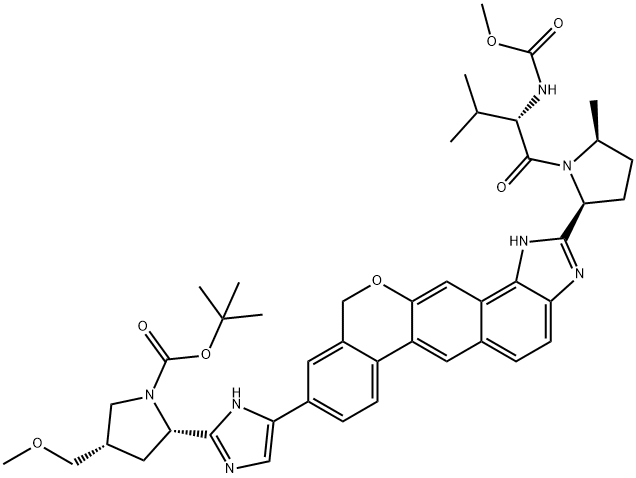
1378391-45-7
110 suppliers
inquiry
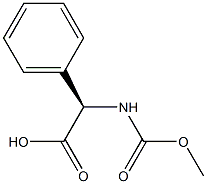
50890-96-5
156 suppliers
$5.00/5g

1377049-84-7
251 suppliers
$9.00/5mg
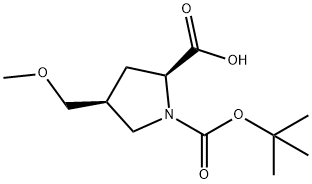
1378388-16-9
193 suppliers
$26.00/250mg

1377049-84-7
251 suppliers
$9.00/5mg
![(2S,4S)-1-tert-butyl 2-(2-oxo-2-(8-oxo-8,9,10,11-tetrahydro-5H-dibenzo[c,g]
chromen-3-yl)ethyl) 4-(methoxymethyl)pyrrolidine-1,2-dicarboxylate](/CAS/20180702/GIF/1378391-39-9.gif)
1378391-39-9
6 suppliers
inquiry

1377049-84-7
251 suppliers
$9.00/5mg
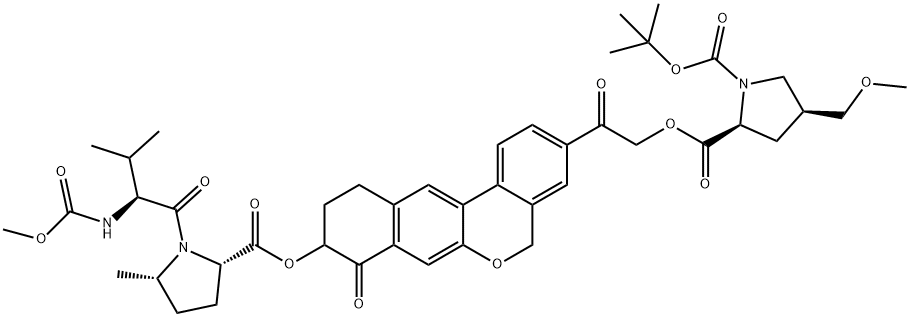
1378391-43-5
35 suppliers
inquiry

1377049-84-7
251 suppliers
$9.00/5mg