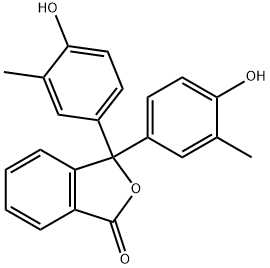
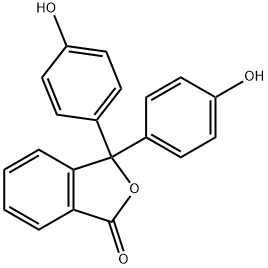
o-Cresolphthalein synthesis
- Product Name:o-Cresolphthalein
- CAS Number:596-27-0
- Molecular formula:C22H18O4
- Molecular Weight:346.38
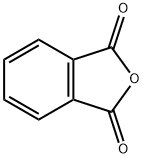
85-44-9
774 suppliers
$9.00/5g
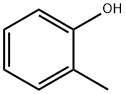
95-48-7
458 suppliers
$18.00/25g
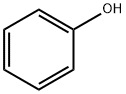
108-95-2
763 suppliers
$14.00/25g
77-09-8
570 suppliers
$10.00/5g
854-76-2
0 suppliers
inquiry

596-27-0
313 suppliers
$17.46/5G
Yield:-
Reaction Conditions:
Stage #1: ortho-cresol;phenolwith zinc(II) chloride in nitrobenzene at 50 - 60;
Stage #2: phthalic anhydridewith zinc(II) chloride in nitrobenzene at 120 - 130; for 4 h;Overall yield = 34 %; Overall yield = 5.9 g;
Steps:
First, we prepared the catalyst based on anhydrousZnCl2. ZnCl2 was melted on a metal spoon over an alcoholburner fl ame. The melt was heated until bubbles ceased toevolve (≈10 min), after which it was cooled, and the cakewas ground in a mortar. The condensation was performedin two steps using nitrobenzene as solvent. First, 4.9 g(0.052 mol) of phenol was added to 18.6 mL (0.18 mol) of nitrobenzene in a metal crucible, after which 5.6 g(0.052 mol) of o-cresol was added, and the mixture washeated to 50-60° and stirred until the phenols dissolvedcompletely. Then, 16.5 g (0.11 mol) of phthalic anhydridewas added, the mixture was stirred, and 14.2 g (0.104 mol)of anhydrous powdered ZnCl2 was added. In the secondstep, the temperature was raised to 120-130°, and themixture was heated for 4 h with intermittent stirring witha putty knife. The following reactant ratio was used in thesynthesis: phthalic anhydride : phenol : ZnCl2 : o-cresol =2.15 : 1.0 : 2.0 : 1.0 ; phenol : nitrobenzene = 1.0 : 3.4. Theproduct yield after purifi cation was 34.0% (5.9 g). Theproduct contained (wt %) 15.0 PP, 34.7 CP, and 50.3 CPP
References:
Nekhoroshev;Nekhoroshev;Poleshchuk;Yarkova;Nekhorosheva;Gasparyan [Russian Journal of Applied Chemistry,2015,vol. 88,# 4,p. 711 - 718][Zh. Prikl. Khim. (S.-Peterburg, Russ. Fed.),2015,vol. 88,# 4,p. 665 - 672,8]