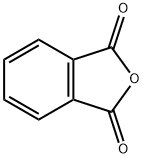
Phthalic anhydride synthesis
- Product Name:Phthalic anhydride
- CAS Number:85-44-9
- Molecular formula:C8H4O3
- Molecular Weight:148.12
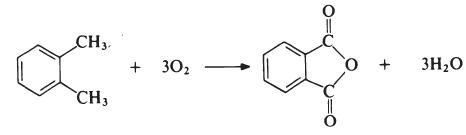
The reaction is carried out in the vapour phase by passing a mixture of o-xylene and air over a catalyst such as vanadium pentoxide supported on silica and promoted with titanium dioxide at about 400°C. The exit gases are cooled and the phthalic anhydride is collected and purified by distillation under reduced pressure.
88-99-3
470 suppliers
$15.00/50g

85-44-9
773 suppliers
$9.00/5g
Yield:85-44-9 100%
Reaction Conditions:
(2-(pyridin-2-yl)phenyl)boronic acid in nonane; for 5.5 h;Product distribution / selectivity;3 ? Molecular sieve;Reflux;
Steps:
8
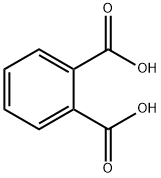
Example 1Phthalic acid (2.5 mmol), 2,6-bis(diisopropylaminomethyl)phenylboronic acid (hereinafter, referred to as “boronic acid compound A”, 0.25 mol) as an arylboronic acid catalyst, and heptane (10 mL) as a solvent were added in a flask having a volume of 20 mL, and a column (small Soxhlet extractor) filled with dried molecular sieves 3A (approximately 3 g) was fitted to the flask. The mixture was heated for 12 hours under azeotropic reflux conditions with the removal of water. After the reaction mixture was cooled to room temperature, heptane was evaporated under reduced pressure. The crude product of phthalic anhydride thus obtained was analyzed by 1H NMR (CDCl3), and the yield thereof was calculated. When the same procedure was repeatedly performed a plurality of times, the yield was 72% to 81%.In addition, the chemical shift (ppm) of 1H NMR was as follows; phthalic acid: δ 7.51-7.60 (m, 2H), and phthalic anhydride: δ 8.05-8.14(m, 2H).Examples 2 to 9, Comparative Examples 1 to 4In Examples 2 to 9 and Comparative Examples 1 to 4, in accordance with Example 1, phthalic anhydride was produced under the conditions shown in Table 1. The results are shown in Table 1. In addition, the result of Example 1 is also collectively shown in Table 1. As apparent from Table 1, in the case of no catalyst, phthalic anhydride was not obtained when heptane having a low boiling point was used as a solvent (Comparative Example 1), and when nonane having a high boiling point was used as a solvent, although phthalic anhydride was obtained, the yield was as low as 12% (Comparative Example 2). In addition, when p-TsOH, the catalyst disclosed in Patent Document 1, was used, although phthalic anhydride was obtained with a high yield of 88% (Comparative Example 3), since this catalyst was a strong acid, for example, corrosion of an iron-based reaction vessel was concerned. Furthermore, when 2,4,6-trimethylphenylboronic acid (having no dialkylaminomethyl group on the ortho position) was used as the catalyst, phthalic anhydride was obtained only in low yield of 7% (Comparative Example 4). On the other hand, when the boronic acid compound A or a derivative thereof having a t-butyl group or a fluorine atom on the para position thereof (referred to as “boronic acid compound B” and “boronic acid compound C”, respectively) was used as the catalyst as in Examples 1 to 6, although the acids mentioned above were each milder than p-TsOH, phthalic anhydride was obtained in good yield (particularly Examples 4 and 5). In addition, when 2-(diisopropylaminomethyl)phenylboronic acid (see J Organometallic Chem., 2005, vol. 690, pp. 4,784 to 4,793) having a dialkylaminomethyl group on one ortho position was used as the catalyst as in Example 7, when a phenylboronic acid having 2-pyridyl group on one ortho position (hereinafter referred to as “boronic acid compound D”) was used as the catalyst as in Example 8, or also when a phenylboronic acid having an ammonium salt which included no hydrogen atoms on one ortho position with one carbon atom provided therebetween (see Tetrahedron, 1999, vol. 55, pp. 2,857 to 2,864, Tetrahedron, 1996, vol. 52, and pp. 12,931 to 12,940.) was used as the catalyst as in Example 9, phthalic anhydride was obtained in high yield. In addition, also when the 2-pyridyl group on the ortho position in Example 8 was replaced with a 3-pyridyl group or an N-methyl-2-imidazolyl group, in the synthesis reaction of phthalic anhydride (however, octane was used as the solvent), activity almost equivalent to that obtained in the case of the 2-pyridyl group was obtained.
References:
US2011/319620,2011,A1 Location in patent:Page/Page column 4-5
87-41-2
481 suppliers
$6.00/10g

85-44-9
773 suppliers
$9.00/5g
6351-10-6
165 suppliers
$6.00/5g

85-44-9
773 suppliers
$9.00/5g
496-14-0
106 suppliers
$43.70/5g

85-44-9
773 suppliers
$9.00/5g
![4,10-DIOXATRICYCLO[5.2.1.0(2,6)]DEC-8-ENE-3,5-DIONE](/CAS/GIF/5426-09-5.gif)
5426-09-5
74 suppliers
inquiry

85-44-9
773 suppliers
$9.00/5g