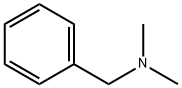
N,N-Dimethylbenzylamine synthesis
- Product Name:N,N-Dimethylbenzylamine
- CAS Number:103-83-3
- Molecular formula:C9H13N
- Molecular Weight:135.21
Benzyl Chloride, 126.6 grams
In the apparatus of Example 1, the benzyl chloride was added dropwise over a two-hour period to the amine (molar ratio 1 to 6) at a rate sufficient to maintain the temperature below 40°C. Stirring was continued at room temperature for an additional hour to insure completion of the reaction denoted by the equation below.
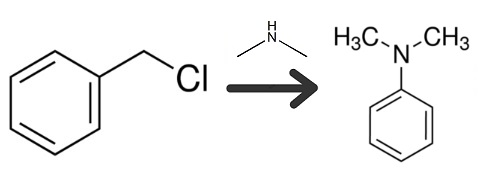
Thereafter the reaction mixture was cooled in a separatory funnel while standing in a refrigerator maintained at 5° C. and separated into two layers. The upper oily layer, weighing 111.5g, was removed and steam distilled until no further oleaginous component was observed in the distillate as it came over. The crude distillate was found to contain 103.5g of N,N-dimethylbenzylamine (76.1% of theory), 3.3g of dimethylamine and no quaternary salts. The dimethylamine was distilled off below 29°C under atmospheric pressure from the N,N-dimethylbenzylamine (bp 82°C/18mmHg).
Yield:103-83-3 99%
Reaction Conditions:
with platinum on carbon;hydrogen;toluene-4-sulfonic acid at 120; under 30003 Torr; for 0.5 h;Inert atmosphere;Autoclave;Reagent/catalyst;
Steps:
16 Examples General procedure for the reductive alkylation of amines with orthocarboxylic acid esters
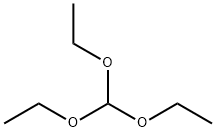
General procedure: An autoclave was filled with catalyst (1 mol% based on the molar amount of amine), flushed with argon and topped up with a solution of amine (0.1 mol) and orthocarboxylic acid ester (0.11-0.3mol) in 10 ml of methanol (or ethanol) and 0.5 ml of a 0.2 M solution of anhydrous ptoluenesulphonic acid in methanol (or ethanol). The mixture was heated to 120°C and hydrogen was injected to 40 bar and then the mixture was stirred at a constant pressure until hydrogen absorption could no longer be detected (0.2 - 6 h). After being filtered off from the catalyst, the filtrate was distilled.
References:
EVONIK DEGUSSA GMBH;KADYROV, Renat WO2017/133913, 2017, A1 Location in patent:Page/Page column 9; 10
124-38-9
130 suppliers
$175.00/23402
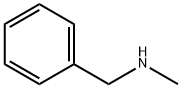
103-67-3
432 suppliers
$5.00/10g

103-83-3
337 suppliers
$14.00/5g
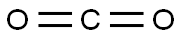
124-38-9
130 suppliers
$175.00/23402
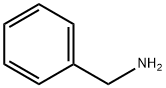
100-46-9
481 suppliers
$5.00/5 g

103-83-3
337 suppliers
$14.00/5g
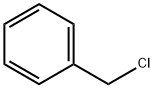
100-44-7
642 suppliers
$13.50/250G
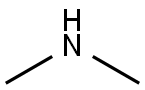
124-40-3
516 suppliers
$18.00/100ml

103-83-3
337 suppliers
$14.00/5g
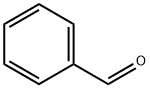
100-52-7
948 suppliers
$17.30/2g

124-40-3
516 suppliers
$18.00/100ml

103-83-3
337 suppliers
$14.00/5g