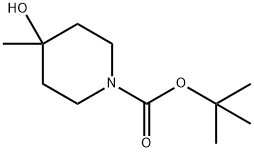
N-BOC-4-METHYL-4-HYDROXY PIPERIDINE synthesis
- Product Name:N-BOC-4-METHYL-4-HYDROXY PIPERIDINE
- CAS Number:406235-30-1
- Molecular formula:C11H21NO3
- Molecular Weight:215.29
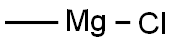
676-58-4
286 suppliers
$15.00/25ml
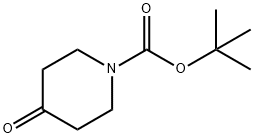
79099-07-3
543 suppliers
$5.00/5g

406235-30-1
118 suppliers
$22.00/250mg
Yield:406235-30-1 99%
Reaction Conditions:
in tetrahydrofuran at -78 - 0; for 2 h;
Steps:
70.A Step A:
tert-butyl 4-hydroxy-4-methylpiperidine-1-carboxylate
To a solution of tert-butyl 4-oxopiperidine-1-carboxylate (1.00 g, 5.02 mmol) in dry THF (25 mL) was added methylmagnesium chloride (2.17 mL, 6.52 mmol) at -78° C.
The reaction mixture was slowly warmed to 0° C. with stirring for 2 hours and quenched with saturated aq. NH4Cl.
The mixture was extracted with EtOAc twice, and the combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo to afford tert-butyl 4-hydroxy-4-methylpiperidine-1-carboxylate (1.08 g, >99%) as a colorless oil, which was used for the next reaction without further purification. 1H-NMR (CDCl3, Varian, 400 MHz): δ 1.27 (3H, s), 1.46 (9H, s), 1.50-1.58 (4H, m), 3.20-3.27 (2H, m), 3.64-3.76 (2H, m).
References:
US2016/168156,2016,A1 Location in patent:Paragraph 0602; 0603
75-16-1
269 suppliers
$12.00/10ml

79099-07-3
543 suppliers
$5.00/5g

406235-30-1
118 suppliers
$22.00/250mg

79099-07-3
543 suppliers
$5.00/5g

406235-30-1
118 suppliers
$22.00/250mg
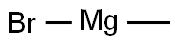
75-16-1
269 suppliers
$12.00/10ml
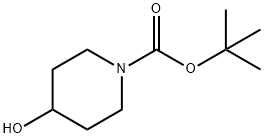
109384-19-2
474 suppliers
$5.00/1g

406235-30-1
118 suppliers
$22.00/250mg
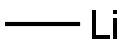
917-54-4
172 suppliers
$52.10/25ml

79099-07-3
543 suppliers
$5.00/5g

406235-30-1
118 suppliers
$22.00/250mg