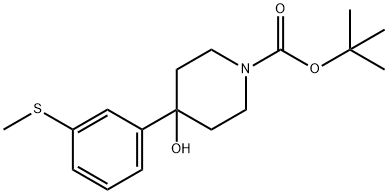
4-Hydroxy-4-(3-methylsulfanylphenyl)-piperidin-1-carboxylic Acid tert-Butyl Ester synthesis
- Product Name:4-Hydroxy-4-(3-methylsulfanylphenyl)-piperidin-1-carboxylic Acid tert-Butyl Ester
- CAS Number:346688-66-2
- Molecular formula:C17H25NO3S
- Molecular Weight:323.45
79099-07-3
543 suppliers
$5.00/5g

346688-66-2
9 suppliers
inquiry
Yield:346688-66-2 76%
Reaction Conditions:
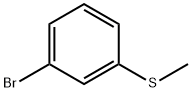
Stage #1: 3-bromo-1-(methylthio)benzenewith n-butyllithium in tetrahydrofuran;hexane at -78 - 0; for 0.583333 h;
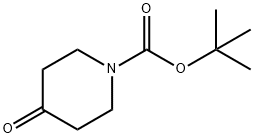
Stage #2: N-tert-butyloxycarbonylpiperidin-4-one in tetrahydrofuran;hexane at 20; for 1 h;
Stage #3: with ammonium chloride in tetrahydrofuran;hexane;
Steps:
1
1-Bromo-3-methylsulfanyl-benzene (5.0 g, 24.6 mmol) was dissolved in dry THF (40 ml) and cooled to -78° C. under a stream of Argon (g). n-BuLi (12.8 ml, 2.5 M in hexane, 31.9 mmol) was added dropwise via syringe and the reaction mixture was stirred for an additional 30 min at -78° C., then the temperature was increased to 0° C. for 5 min and then decreased to -78° C. 1-tert-Butoxycarbonyl-4-piperidone (5.4 g, 27.06 mmol) dissolved in dry THF (30 ml) was added via syringe. The reaction mixture was allowed to reach room temperature and then stirred for 1 hour, and finally quenched with saturated ammonium chloride solution (30 ml). The mixture was extracted several times with EtOAc and the combined organic phases were dried (MgSO4), filtered and evaporated to dryness. The oily residue was chromatho-graphed on a silica column using CH2Cl2:MeOH (19:1 (v/v)) as eluent, yielded 4-hydroxy-4-(3-methylsulfanyl-phenyl)-piperidin-1-carboxylic acid tert-butyl ester (6 g, 76%). MS m/z (relative intensity, 70-eV) 323.1 (M+, 6), 223.0 (11), 178.0 (7), 152 (3), 57.0 (bp), 56 (30).
References:
US2006/135531,2006,A1 Location in patent:Page/Page column 13