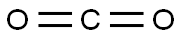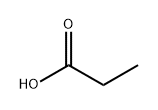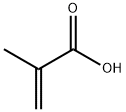
Methacrylic acid synthesis
- Product Name:Methacrylic acid
- CAS Number:79-41-4
- Molecular formula:C4H6O2
- Molecular Weight:86.09
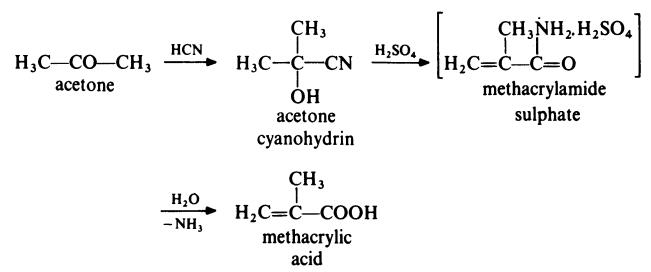
In a typical process, acetone is treated with hydrogen cyanide at 140°C in the presence of ammonia as catalyst. The acetone cyanohydrin produced is treated with concentrated sulphuric acid at 100°C to form methacrylamide sulphate. This intermediate is not isolated but is directly converted to methacrylic acid by treatment with water at about 90°C.
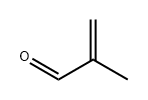
A competitive route now in commercial operation involves the two stage oxidation of isobutene with air. The reaction proceeds via methacrolein:
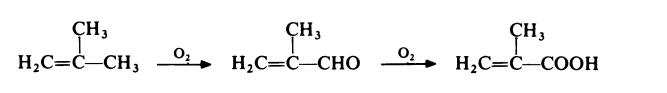
Yield:79-41-4 87%
Reaction Conditions:
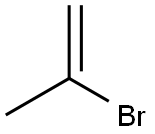
Stage #1: 2-bromoprop-1-enewith magnesium in tetrahydrofuran at 20; for 0.5 h;
Stage #2: carbon dioxide in tetrahydrofuran at -70 - 20; under 750.075 Torr;
Stage #3: with hydrogenchloride;ammonium chlorideProduct distribution / selectivity;more than 3 stages;
Steps:
1.a
Synthesis of methacrylic acid, a compound of formula (II), byGrignard reaction starting from 2-bromopropene, a compound of formula (III)2-Bromopropene (24.20 g, 200 mmol) dissolved in tetrahydrofuran (THF, 100 ml) was added drop wise to a stirred suspension of magnesium turnings (4.38 g, 180 mmol) in THF (100 ml) under nitrogen-atmosphere. The 2-bromopropene was added at a rate causing the reaction mixture to boil gently. After complete formation of the Grignard reagent had been achieved, the mixture was stirred at room temperature for 30 min, before being cooled to - 70 0C. The reaction mixture was subjected to an atmosphere of carbon dioxide (1 bar) under heavy stirring (vortexing). The reaction was allowed to reach room temperature and then terminated by addition of saturated aqueous ammonium chloride (100 ml). THF and excess 2-bromopropene was removed in vacuo at 30 °C and the aqueous phase was acidified by addition of aqueous HCl (3M, 50 ml) before extraction with dichloromethane (3 x 50 ml). The combined organic extracts were dried over magnesium sulfate and evaporated to give the title compound; a colourless or slightly yellow oil. Yield: 13.50 g (87 %), purity 99%.
References:
WO2008/48106,2008,A1 Location in patent:Page/Page column 17
78-85-3
175 suppliers
$56.90/5ml

79-41-4
501 suppliers
$14.00/5g
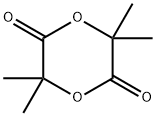
6713-72-0
6 suppliers
inquiry

79-41-4
501 suppliers
$14.00/5g
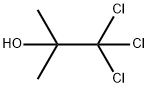
57-15-8
245 suppliers
inquiry

79-41-4
501 suppliers
$14.00/5g